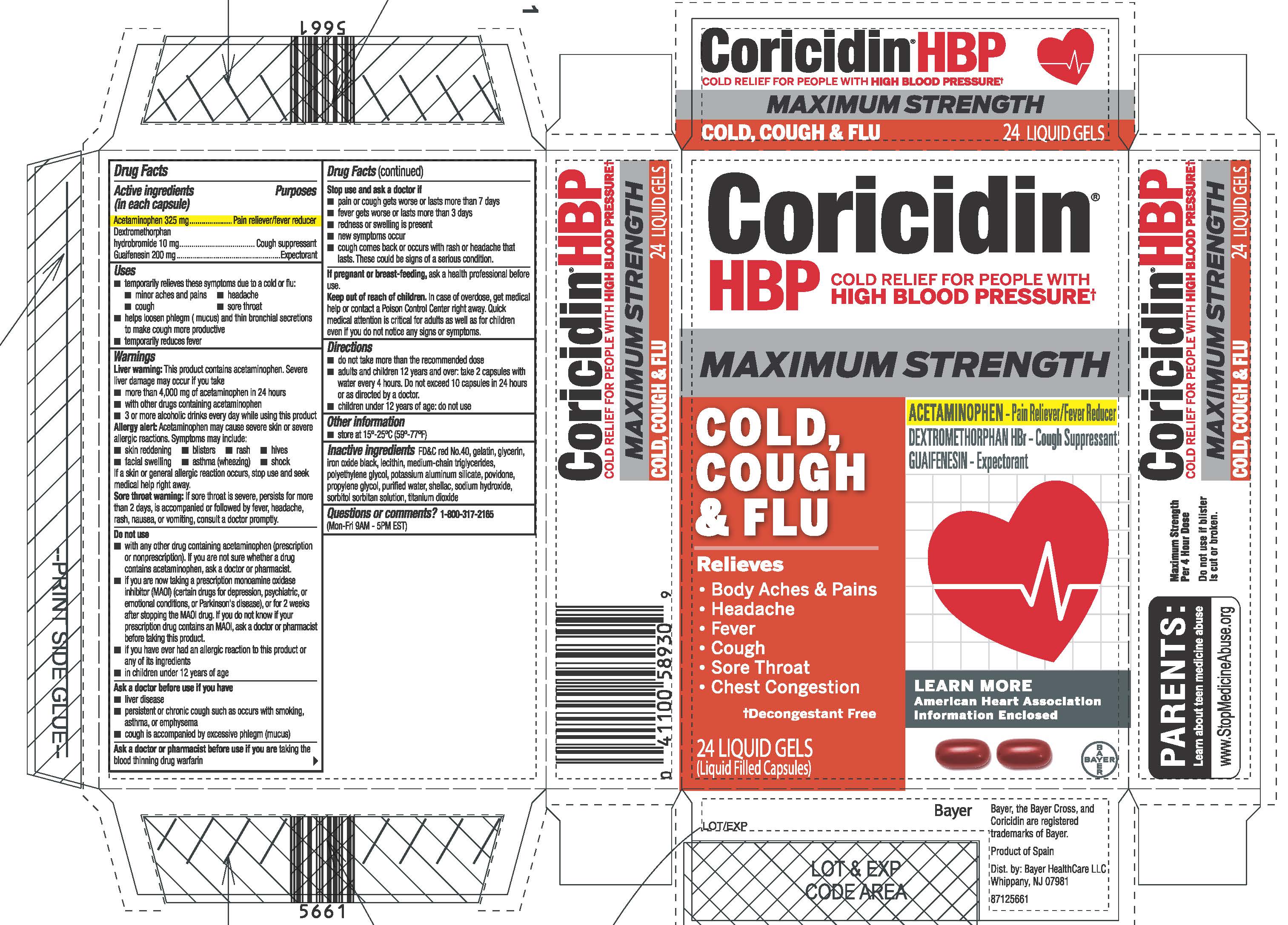
Coricidin HBP Maximum Strength Cold, Cough & Flu Liquid Gels, UI1614532
Coricidin HBP Maximum Strength Cold, Cough and Flu by
Drug Labeling and Warnings
Coricidin HBP Maximum Strength Cold, Cough and Flu by is a Otc medication manufactured, distributed, or labeled by Bayer HealthCare LLC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CORICIDIN HBP MAXIMUM STRENGTH COLD, COUGH AND FLU- acetaminophen, dextromethorphan hydrobromide, guaifenesin capsule, liquid filled
Bayer HealthCare LLC.
----------
Coricidin HBP Maximum Strength Cold, Cough & Flu Liquid Gels, UI1614532
Active Ingredients
Active ingredients (in each capsule) Purposes
Acetaminophen 325 mg…………….………..Pain reliever/fever reducer
Dextromethorphan hydrobromide 10 mg…………..Cough suppressant
Guaifenesin 200 mg…………………………………………...Expectorant
Uses
Uses
· temporarily relieves these symptoms due to a cold or flu: · minor aches and pains · headache · cough · sore throat
· helps loosen phlegm ( mucus) and thin bronchial secretions to make cough more productive
· temporarily reduces fever
Warnings
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
· more than 4,000 mg of acetaminophen in 24 hours
· with other drugs containing acetaminophen
· 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin or severe
allergic reactions. Symptoms may include:
· skin reddening · blisters · rash · hives
· facial swelling · asthma (wheezing) · shock
If a skin or general allergic reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than
2 days, is accompanied or followed by fever, headache, rash, nausea,
or vomiting, consult a doctor promptly.
Do not use
Do not use
● with any other drug containing acetaminophen (prescription or
nonprescription). If you are not sure whether a drug contains
acetaminophen, ask a doctor or pharmacist.
● if you are now taking a prescription monoamine oxidase inhibitor
(MAOI) (certain drugs for depression, psychiatric, or emotional
conditions, or Parkinson's disease), or for 2 weeks after stopping
the MAOI drug. If you do not know if your prescription drug contains
an MAOI, ask a doctor or pharmacist before taking this product.
● if you have ever had an allergic reaction to this product or any of its
ingredients
● in children under 12 years of age
Ask a doctor before use if you have
Ask a doctor before use if you have
● liver disease
● persistent or chronic cough such as occurs with smoking, asthma,
or emphysema
● cough is accompanied by excessive phlegm (mucus)
Ask a doctor or pharmacist
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
Stop use and ask a doctor if
Stop use and ask a doctor if
· pain or cough gets worse or lasts more than 7 days
· fever gets worse or lasts more than 3 days
· redness or swelling is present
· new symptoms occur
· cough comes back or occurs with rash or headache that lasts.
These could be signs of a serious condition.
Directions
Directions
· do not take more than the recommended dose
· adults and children 12 years and over: take 2 capsules with water
every 4 hours. Do not exceed 10 capsules in 24 hours or as
directed by a doctor.
· children under 12 years of age: do not use
| CORICIDIN HBP MAXIMUM STRENGTH COLD, COUGH AND FLU
acetaminophen, dextromethorphan hydrobromide, guaifenesin capsule, liquid filled |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.