XYZAL- levocetirizine dihydrochloride tablet, film coated XYZAL- levocetirizine dihydrochloride solution
Xyzal by
Drug Labeling and Warnings
Xyzal by is a Prescription medication manufactured, distributed, or labeled by Sanofi-Aventis U.S. LLC, UCB Farchim S.A., Bausch Health Companies Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use XYZAL® safely and effectively. See full prescribing information for XYZAL.
XYZAL (levocetirizine dihydrochloride) tablets, for oral use
XYZAL (levocetirizine dihydrochloride) oral solution
Initial U.S. Approval: 1995INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Perennial Allergic Rhinitis (2.1)
- Children 6 months to 2 years of age: 1.25 mg (1/2 teaspoon oral solution) (2.5 mL) once daily in the evening
Chronic Idiopathic Urticaria (2.2)
- Adults and children 12 years of age and older: 5 mg once daily in the evening
- Children 6 to 11 years of age: 2.5 mg once daily in the evening
- Children 6 months to 5 years of age: 1.25 mg (1/2 teaspoon oral solution) (2.5 mL) once daily in the evening
- Renal Impairment
Adjust the dose in patients 12 years of age and older with decreased renal function (12.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- Patients with a known hypersensitivity to levocetirizine or any of the ingredients of XYZAL or to cetirizine (4.1)
- Patients with end-stage renal disease at less than 10 mL/min creatinine clearance or patients undergoing hemodialysis (4.2)
- Children 6 months to 11 years of age with renal impairment (4.3)
WARNINGS AND PRECAUTIONS
- Avoid engaging in hazardous occupations requiring complete mental alertness such as driving or operating machinery when taking XYZAL. (5.1)
- Avoid concurrent use of alcohol or other central nervous system depressants with XYZAL. (5.1)
- Use with caution in patients with predisposing factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia). Discontinue XYZAL if urinary retention occurs. (5.2)
ADVERSE REACTIONS
The most common adverse reactions (rate ≥2% and > placebo) were somnolence, nasopharyngitis, fatigue, dry mouth, and pharyngitis in subjects 12 years of age and older, and pyrexia, somnolence, cough, and epistaxis in children 6 to 12 years of age. In subjects 1 to 5 years of age, the most common adverse reactions (rate ≥2% and > placebo) were pyrexia, diarrhea, vomiting, and otitis media. In subjects 6 to 11 months of age, the most common adverse reactions (rate ≥3% and > placebo) were diarrhea and constipation. (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis U.S. LLC at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Renal Impairment
Because XYZAL is substantially excreted by the kidneys, the risk of adverse reactions to this drug may be greater in patients with impaired renal function. (8.6, 12.3) - Pediatric Use
Do not exceed the recommended doses of 2.5 mg and 1.25 mg once daily in children 6 to 11 years and 6 months to 5 years of age, respectively. Systemic exposure with these doses in respective pediatric age groups is comparable to that from a 5 mg once daily dose in adults. (12.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Perennial Allergic Rhinitis
1.2 Chronic Idiopathic Urticaria
2 DOSAGE AND ADMINISTRATION
2.1 Perennial Allergic Rhinitis
2.2 Chronic Idiopathic Urticaria
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Patients with Known Hypersensitivity
4.2 Patients with End-Stage Renal Disease
4.3 Pediatric Patients with Impaired Renal Function
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence
5.2 Urinary Retention
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Antipyrine, Azithromycin, Cimetidine, Erythromycin, Ketoconazole, Theophylline, and Pseudoephedrine
7.2 Ritonavir
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Perennial Allergic Rhinitis
14.2 Chronic Idiopathic Urticaria
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
XYZAL is available as 2.5 mg/5 mL (0.5 mg/mL) oral solution and as 5 mg breakable (scored) tablets, allowing for the administration of 2.5 mg, if needed. XYZAL can be taken without regard to food consumption.
2.1 Perennial Allergic Rhinitis
Children 6 months to 2 Years of Age
The recommended initial dose of XYZAL is 1.25 mg (1/2 teaspoon oral solution) (2.5 mL) once daily in the evening. The 1.25 mg once daily dose should not be exceeded based on comparable exposure to adults receiving 5 mg [see Clinical Pharmacology (12.3)].
2.2 Chronic Idiopathic Urticaria
Adults and Children 12 Years of Age and Older
The recommended dose of XYZAL is 5 mg (1 tablet or 2 teaspoons [10 mL] oral solution) once daily in the evening. Some patients may be adequately controlled by 2.5 mg (1/2 tablet or 1 teaspoon [5 mL] oral solution) once daily in the evening.
Children 6 to 11 Years of Age
The recommended dose of XYZAL is 2.5 mg (1/2 tablet or 1 teaspoon [5 mL] oral solution) once daily in the evening. The 2.5 mg dose should not be exceeded because the systemic exposure with 5 mg is approximately twice that of adults [see Clinical Pharmacology (12.3)].
Children 6 months to 5 Years of Age
The recommended initial dose of XYZAL is 1.25 mg (1/2 teaspoon oral solution) (2.5 mL) once daily in the evening. The 1.25 mg once daily dose should not be exceeded based on comparable exposure to adults receiving 5 mg [see Clinical Pharmacology (12.3)].
Dose Adjustment for Renal and Hepatic Impairment
In adults and children 12 years of age and older with:
- Mild renal impairment (creatinine clearance [CLCR] = 50–80 mL/min): a dose of 2.5 mg once daily is recommended;
- Moderate renal impairment (CLCR = 30–50 mL/min): a dose of 2.5 mg once every other day is recommended;
- Severe renal impairment (CLCR = 10–30 mL/min): a dose of 2.5 mg twice weekly (administered once every 3–4 days) is recommended;
- End-stage renal disease patients (CLCR <10 mL/min) and patients undergoing hemodialysis should not receive XYZAL.
No dose adjustment is needed in patients with solely hepatic impairment. In patients with both hepatic impairment and renal impairment, adjustment of the dose is recommended.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
The use of XYZAL is contraindicated in:
4.1 Patients with Known Hypersensitivity
Patients with known hypersensitivity to levocetirizine or any of the ingredients of XYZAL, or to cetirizine. Observed reactions range from urticaria to anaphylaxis [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence
In clinical trials the occurrence of somnolence, fatigue, and asthenia has been reported in some patients under therapy with XYZAL. Patients should be cautioned against engaging in hazardous occupations requiring complete mental alertness, and motor coordination such as operating machinery or driving a motor vehicle after ingestion of XYZAL. Concurrent use of XYZAL with alcohol or other central nervous system depressants should be avoided because additional reductions in alertness and additional impairment of central nervous system performance may occur.
5.2 Urinary Retention
Urinary retention has been reported post marketing with XYZAL. XYZAL should be used with caution in patients with predisposing factors of urinary retention (e.g. spinal cord lesion, prostatic hyperplasia) as XYZAL may increase the risk of urinary retention. Discontinue XYZAL if urinary retention occurs.
-
6 ADVERSE REACTIONS
Use of XYZAL has been associated with somnolence, fatigue, asthenia, and urinary retention [see Warnings and Precautions (5)].
6.1 Clinical Trials Experience
The safety data described below reflect exposure to XYZAL in 2708 patients with allergic rhinitis or chronic idiopathic urticaria in 14 controlled clinical trials of 1 week to 6 months duration.
The short-term (exposure up to 6 weeks) safety data for adults and adolescents are based upon eight clinical trials in which 1896 patients (825 males and 1071 females aged 12 years and older) were treated with XYZAL 2.5, 5, or 10 mg once daily in the evening.
The short-term safety data from pediatric patients are based upon two clinical trials in which 243 children with allergic rhinitis (162 males and 81 females 6 to 12 years of age) were treated with XYZAL 5 mg once daily for 4 to 6 weeks, one clinical trial in which 114 children (65 males and 49 females 1 to 5 years of age) with allergic rhinitis or chronic idiopathic urticaria were treated with XYZAL 1.25 mg twice daily for 2 weeks, and one clinical trial in which 45 children (28 males and 17 females 6 to 11 months of age) with symptoms of allergic rhinitis or chronic urticaria were treated with XYZAL 1.25 mg once daily for 2 weeks.
The long-term (exposure of 4 or 6 months) safety data in adults and adolescents are based upon two clinical trials in which 428 patients (190 males and 238 females) with allergic rhinitis were exposed to treatment with XYZAL 5 mg once daily. Long term safety data are also available from an 18-month trial in 255 XYZAL-treated subjects 12–24 months of age.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trial of another drug and may not reflect the rates observed in practice.
Adults and Adolescents 12 years of Age and Older
In studies up to 6 weeks in duration, the mean age of the adult and adolescent patients was 32 years, 44% of the patients were men and 56% were women, and the large majority (more than 90%) was Caucasian.
In these trials 43% and 42% of the subjects in the XYZAL 2.5 mg and 5 mg groups, respectively, had at least one adverse event compared to 43% in the placebo group.
In placebo-controlled trials of 1–6 weeks in duration, the most common adverse reactions were somnolence, nasopharyngitis, fatigue, dry mouth, and pharyngitis, and most were mild to moderate in intensity. Somnolence with XYZAL showed dose ordering between tested doses of 2.5, 5 and 10 mg and was the most common adverse reaction leading to discontinuation (0.5%).
Table 1 lists adverse reactions that were reported in greater than or equal to 2% of subjects aged 12 years and older exposed to XYZAL 2.5 mg or 5 mg in eight placebo-controlled clinical trials and that were more common with XYZAL than placebo.
Table 1: Adverse Reactions Reported in ≥2%* of Subjects Aged 12 Years and Older Exposed to XYZAL 2.5 mg or 5 mg Once Daily in Placebo-Controlled Clinical Trials 1–6 Weeks in Duration Adverse Reactions XYZAL 2.5 mg
(n = 421)XYZAL 5 mg
(n = 1070)Placebo
(n = 912)- * Rounded to the closest unit percentage
Somnolence 22 (5%) 61 (6%) 16 (2%) Nasopharyngitis 25 (6%) 40 (4%) 28 (3%) Fatigue 5 (1%) 46 (4%) 20 (2%) Dry Mouth 12 (3%) 26 (2%) 11 (1%) Pharyngitis 10 (2%) 12 (1%) 9 (1%) Additional adverse reactions of medical significance observed at a higher incidence than in placebo in adults and adolescents aged 12 years and older exposed to XYZAL are syncope (0.2%) and weight increased (0.5%).
Pediatric Patients 6 to 12 Years of Age
A total of 243 pediatric patients 6 to 12 years of age received XYZAL 5 mg once daily in two short-term placebo controlled double-blind trials. The mean age of the patients was 9.8 years, 79 (32%) were 6 to 8 years of age, and 50% were Caucasian. Table 2 lists adverse reactions that were reported in greater than or equal to 2% of subjects aged 6 to 12 years exposed to XYZAL 5 mg in placebo-controlled clinical trials and that were more common with XYZAL than placebo.
Table 2: Adverse Reactions Reported in ≥2%* of Subjects Aged 6–12 Years Exposed to XYZAL 5 mg Once Daily in Placebo-Controlled Clinical Trials 4 and 6 Weeks in Duration Adverse Reactions XYZAL 5 mg
(n = 243)Placebo
(n = 240)- * Rounded to the closest unit percentage
Pyrexia 10 (4%) 5 (2%) Cough 8 (3%) 2 (<1%) Somnolence 7 (3%) 1 (<1%) Epistaxis 6 (2%) 1 (<1%) Pediatric Patients 1 to 5 Years of Age
A total of 114 pediatric patients 1 to 5 years of age received XYZAL 1.25 mg twice daily in a two week placebo-controlled double-blind safety trial. The mean age of the patients was 3.8 years, 32% were 1 to 2 years of age, 71% were Caucasian and 18% were Black. Table 3 lists adverse reactions that were reported in greater than or equal to 2% of subjects aged 1 to 5 years exposed to XYZAL 1.25 mg twice daily in the placebo-controlled safety trial and that were more common with XYZAL than placebo.
Table 3: Adverse Reactions Reported in ≥2%* of Subjects Aged 1–5 Years Exposed to XYZAL 1.25 mg Twice Daily in a 2-Week Placebo-Controlled Clinical Trial Adverse Reactions XYZAL 1.25 mg Twice Daily
(n = 114)Placebo
(n = 59)- * Rounded to the closest unit percentage
Pyrexia 5 (4%) 1 (2%) Diarrhea 4 (4%) 2 (3%) Vomiting 4 (4%) 2 (3%) Otitis Media 3 (3%) 0 (0%) Pediatric Patients 6 to 11 Months of Age
A total of 45 pediatric patients 6 to 11 months of age received XYZAL 1.25 mg once daily in a two week placebo-controlled double-blind safety trial. The mean age of the patients was 9 months, 51% were Caucasian and 31% were Black. Adverse reactions that were reported in more than 1 subject (i.e. greater than or equal to 3% of subjects) aged 6 to 11 months exposed to XYZAL 1.25 mg once daily in the placebo-controlled safety trial and that were more common with XYZAL than placebo included diarrhea and constipation which were reported in 6 (13%) and 1 (4%) and 3 (7%) and 1 (4%) children in the XYZAL and placebo-treated groups, respectively.
Long-Term Clinical Trials Experience
In two controlled clinical trials, 428 patients (190 males and 238 females) aged 12 years and older were treated with XYZAL 5 mg once daily for 4 or 6 months. The patient characteristics and the safety profile were similar to that seen in the short-term studies. Ten (2.3%) patients treated with XYZAL discontinued because of somnolence, fatigue or asthenia compared to 2 (<1%) in the placebo group.
There are no long term clinical trials in children below 12 years of age with allergic rhinitis or chronic idiopathic urticaria.
6.2 Postmarketing Experience
In addition to the adverse reactions reported during clinical trials and listed above, the following adverse reactions have also been identified during postapproval use of XYZAL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiac disorders: palpitations, tachycardia
- Ear and labyrinth disorders: vertigo
- Eye disorders: blurred vision, visual disturbances
- Gastrointestinal disorders: nausea, vomiting
- General disorders and administration site conditions: edema
- Hepatobiliary disorders: hepatitis
- Immune system disorders: anaphylaxis and hypersensitivity
- Metabolism and nutrition disorders: increased appetite
- Musculoskeletal, connective tissues, and bone disorders: arthralgia, myalgia
- Nervous system disorders: dizziness, dysgeusia, febrile seizure, movement disorders (including dystonia and oculogyric crisis), paresthesia, seizure (reported in subjects with and without a known seizure disorder), tremor
- Psychiatric disorders: aggression and agitation, depression, hallucinations, insomnia, nightmare, suicidal ideation
- Renal and urinary disorders: dysuria, urinary retention
- Respiratory, thoracic, and mediastinal disorders: dyspnea
- Skin and subcutaneous tissue disorders: angioedema, fixed drug eruption, pruritus, rash and urticaria
Besides these reactions reported under treatment with XYZAL, other potentially severe adverse events have been reported from the postmarketing experience with cetirizine. Since levocetirizine is the principal pharmacologically active component of cetirizine, one should take into account the fact that the following adverse events could also potentially occur under treatment with XYZAL.
- Cardiac disorders: severe hypotension
- Gastrointestinal disorders: cholestasis
- Nervous system disorders: extrapyramidal symptoms, myoclonus, orofacial dyskinesia, tic
- Pregnancy, puerperium and perinatal conditions: stillbirth
- Renal and urinary disorders: glomerulonephritis
- Skin and subcutaneous tissue disorders: acute generalized exanthematous pustulosis (AGEP); rebound pruritus - pruritus within a few days after discontinuation of cetirizine, usually after long-term use (e.g. months to years) of cetirizine.
-
7 DRUG INTERACTIONS
In vitro data indicate that levocetirizine is unlikely to produce pharmacokinetic interactions through inhibition or induction of liver drug-metabolizing enzymes. No in vivo drug-drug interaction studies have been performed with levocetirizine. Drug interaction studies have been performed with racemic cetirizine.
7.1 Antipyrine, Azithromycin, Cimetidine, Erythromycin, Ketoconazole, Theophylline, and Pseudoephedrine
Pharmacokinetic interaction studies performed with racemic cetirizine demonstrated that cetirizine did not interact with antipyrine, pseudoephedrine, erythromycin, azithromycin, ketoconazole, and cimetidine. There was a small decrease (~16%) in the clearance of cetirizine caused by a 400 mg dose of theophylline. It is possible that higher theophylline doses could have a greater effect.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published literature and postmarketing experience with levocetirizine use in pregnant women are insufficient to identify any drug-associated risks of miscarriage, birth defects, or adverse maternal or fetal outcomes. In animal reproduction studies, there was no evidence of fetal harm with administration of levocetirizine by the oral route to pregnant rats and rabbits, during the period of organogenesis, at doses up to 390 times and 470 times, respectively, the maximum recommended human dose (MRHD) in adults. In rats treated during late gestation and the lactation period, cetirizine had no effects on pup development at oral doses up to approximately 60 times the MRHD in adults. In mice treated during late gestation and the lactation period, cetirizine administered by the oral route to the dams had no effects on pup development at a dose that was approximately 25 times the MRHD in adults; however, lower pup weight gain during lactation was observed at a dose that was 95 times the MRHD in adults [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal data
In embryo-fetal development studies, pregnant rats received daily doses of levocetirizine up to 200 mg/kg/day from gestation days 6 to 15 and pregnant rabbits received daily doses of levocetirizine up to 120 mg/kg/day from gestation days 6 to 18. Levocetirizine produced no evidence of fetal harm in rats and rabbits at doses up to 390 and 470 times the MRHD, respectively (on a mg/m2 basis with maternal oral doses of 200 and 120 mg/kg/day in rats and rabbits, respectively).
No prenatal and postnatal development (PPND) studies in animals have been conducted with levocetirizine. In a PPND study conducted in mice, cetirizine was administered at oral doses up to 96 mg/kg/day from gestation day 15 through lactation day 21. Cetirizine lowered pup body weight gain during lactation at an oral dose in dams that was approximately 95 times the MRHD (on a mg/m2 basis with a maternal oral dose of 96 mg/kg/day); however, there were no effects on pup weight gain at an oral dose in dams that was approximately 25 times the MRHD (on a mg/m2 basis with a maternal oral dose of 24 mg/kg/day). In a PPND study conducted in rats, cetirizine was administered at oral doses up to 180 mg/kg/day from gestation day 17 to lactation day 22. Cetirizine did not have any adverse effects on rat dams or offspring development at doses up to approximately 60 times the MRHD (on a mg/m2 basis with a maternal oral dose of 30 mg/kg/day). Cetirizine caused excessive maternal toxicity at an oral dose in dams that was approximately 350 times the MRHD (on a mg/m2 basis with a maternal oral dose of 180 mg/kg/day).
8.2 Lactation
Risk Summary
There are no data on the presence of levocetirizine in human milk, the effects on the breastfed infant, or the effects on milk production. However, cetirizine has been reported to be present in human breast milk. In mice and beagle dogs, studies indicated that cetirizine was excreted in milk [see Data]. When a drug is present in animal milk, it is likely the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for XYZAL and any potential adverse effects on the breastfed child from XYZAL or from the underlying maternal condition.
Data
Animal data
Cetirizine was detected in the milk of mice. No adverse developmental effects on pups were seen when cetirizine was administered orally to dams during lactation at a dose that was approximately 25 times the MRHD in adults [see Use in Specific Populations (8.1)]. Studies in beagle dogs indicated that approximately 3% of the dose of cetirizine was excreted in milk. The concentration of drug in animal milk does not necessarily predict the concentration of drug in human milk.
8.4 Pediatric Use
The recommended dose of XYZAL for the treatment of the uncomplicated skin manifestations of chronic idiopathic urticaria in patients 6 months to 17 years of age is based on extrapolation of efficacy from adults 18 years of age and older [see Clinical Studies (14)].
The recommended dose of XYZAL in patients 6 months to 2 years of age for the treatment of the symptoms of perennial allergic rhinitis and 6 months to 11 years of age with chronic idiopathic urticaria is based on cross-study comparisons of the systemic exposure of XYZAL in adults and pediatric patients and on the safety profile of XYZAL in both adult and pediatric patients at doses equal to or higher than the recommended dose for patients 6 months to 11 years of age.
The safety of XYZAL 5 mg once daily was evaluated in 243 pediatric patients 6 to 12 years of age in two placebo-controlled clinical trials lasting 4 and 6 weeks. The safety of XYZAL 1.25 mg twice daily was evaluated in one 2-week clinical trial in 114 pediatric patients 1 to 5 years of age and the safety of XYZAL 1.25 mg once daily was evaluated in one 2-week clinical trial in 45 pediatric patients 6 to 11 months of age [see Adverse Reactions (6.1)].
The effectiveness of XYZAL 1.25 mg once daily (6 months to 5 years of age) and 2.5 mg once daily (6 to 11 years of age) for the treatment of the symptoms of perennial allergic rhinitis and chronic idiopathic urticaria is supported by the extrapolation of demonstrated efficacy of XYZAL 5 mg once daily in patients 12 years of age and older based on the pharmacokinetic comparison between adults and children.
Cross-study comparisons indicate that administration of a 5 mg dose of XYZAL to 6 to 12 year old pediatric patients resulted in about 2-fold the systemic exposure (AUC) observed when 5 mg of XYZAL was administered to healthy adults. Therefore, in children 6 to 11 years of age the recommended dose of 2.5 mg once daily should not be exceeded. In a population pharmacokinetics study the administration of 1.25 mg once daily in children 6 months to 5 years of age resulted in systemic exposure comparable to 5 mg once daily in adults. [see Dosage and Administration (2.2), Clinical Studies (14), and Clinical Pharmacology (12.3)].
8.5 Geriatric Use
Clinical studies of XYZAL for each approved indication did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
8.6 Renal Impairment
XYZAL is known to be substantially excreted by the kidneys and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function [see Dosage and Administration (2) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
As levocetirizine is mainly excreted unchanged by the kidneys, it is unlikely that the clearance of levocetirizine is significantly decreased in patients with solely hepatic impairment [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Overdosage has been reported with XYZAL.
Symptoms of overdose may include drowsiness in adults. In children agitation and restlessness may initially occur, followed by drowsiness. There is no known specific antidote to XYZAL. Should overdose occur, symptomatic or supportive treatment is recommended. XYZAL is not effectively removed by dialysis, and dialysis will be ineffective unless a dialyzable agent has been concomitantly ingested.
The acute maximal non-lethal oral dose of levocetirizine was 240 mg/kg in mice (approximately 190 times the maximum recommended daily oral dose in adults, approximately 230 times the maximum recommended daily oral dose in children 6 to 11 years of age, and approximately 180 times the maximum recommended daily oral dose in children 6 months to 5 years of age on a mg/m2 basis). In rats the maximal non-lethal oral dose was 240 mg/kg (approximately 390 times the maximum recommended daily oral dose in adults, approximately 460 times the maximum recommended daily oral dose in children 6 to 11 years of age, and approximately 370 times the maximum recommended daily oral dose in children 6 months to 5 years of age on a mg/m2 basis).
-
11 DESCRIPTION
Levocetirizine dihydrochloride, the active component of XYZAL tablets and oral solution, is an orally active H1-receptor antagonist. The chemical name is (R)-[2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl] ethoxy] acetic acid dihydrochloride. Levocetirizine dihydrochloride is the R enantiomer of cetirizine hydrochloride, a racemic compound with antihistaminic properties. The empirical formula of levocetirizine dihydrochloride is C21H25ClN2O3∙2HCl. The molecular weight is 461.82 and the chemical structure is shown below:
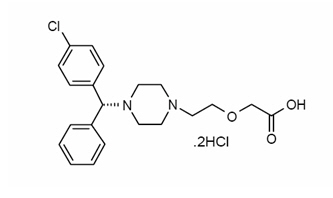
Levocetirizine dihydrochloride is a white, crystalline powder and is water soluble.
XYZAL 5 mg tablets are formulated as immediate release, white, film-coated, oval-shaped scored tablets for oral administration. The tablets are imprinted on both halves of the scored line with the letter Y in red (Opacode® Red). Inactive ingredients are: microcrystalline cellulose, lactose monohydrate, colloidal anhydrous silica, and magnesium stearate. The film coating contains hypromellose, titanium dioxide, and macrogol 400.
XYZAL 0.5 mg/mL oral solution is formulated as an immediate release, clear, colorless liquid. Inactive ingredients are: sodium acetate trihydrate, glacial acetic acid, maltitol solution, glycerin, methylparaben, propylparaben, saccharin, flavoring (consisting of triacetin, natural & artificial flavors, dl-alpha-tocopherol), purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Levocetirizine, the active enantiomer of cetirizine, is an antihistamine; its principal effects are mediated via selective inhibition of H1 receptors. The antihistaminic activity of levocetirizine has been documented in a variety of animal and human models. In vitro binding studies revealed that levocetirizine has an affinity for the human H1-receptor 2-fold higher than that of cetirizine (Ki = 3 nmol/L vs. 6 nmol/L, respectively). The clinical relevance of this finding is unknown.
12.2 Pharmacodynamics
Studies in adult healthy subjects showed that levocetirizine at doses of 2.5 mg and 5 mg inhibited the skin wheal and flare caused by the intradermal injection of histamine. In contrast, dextrocetirizine exhibited no clear change in the inhibition of the wheal and flare reaction. Levocetirizine at a dose of 5 mg inhibited the wheal and flare caused by intradermal injection of histamine in 14 pediatric subjects (aged 6 to 11 years) and the activity persisted for at least 24 hours. The clinical relevance of histamine wheal skin testing is unknown.
A QT/QTc study using a single dose of 30 mg of levocetirizine did not demonstrate an effect on the QTc interval. While a single dose of levocetirizine had no effect, the effects of levocetirizine may not be at steady state following single dose. The effect of levocetirizine on the QTc interval following multiple dose administration is unknown. Levocetirizine is not expected to have QT/QTc effects because of the results of QTc studies with cetirizine and the long postmarketing history of cetirizine without reports of QT prolongation.
12.3 Pharmacokinetics
Levocetirizine exhibited linear pharmacokinetics over the therapeutic dose range in adult healthy subjects.
Absorption
Levocetirizine is rapidly and extensively absorbed following oral administration. In adults, peak plasma concentrations are achieved 0.9 hour after administration of the oral tablet. The accumulation ratio following daily oral administration is 1.12 with steady state achieved after 2 days. Peak concentrations are typically 270 ng/mL and 308 ng/mL following a single and a repeated 5 mg once daily dose, respectively. Food had no effect on the extent of exposure (AUC) of the levocetirizine tablet, but Tmax was delayed by about 1.25 hours and Cmax was decreased by about 36% after administration with a high fat meal; therefore, levocetirizine can be administered with or without food.
A dose of 5 mg (10 mL) of XYZAL oral solution is bioequivalent to a 5 mg dose of XYZAL tablets. Following oral administration of a 5 mg dose of XYZAL oral solution to healthy adult subjects, the mean peak plasma concentrations were achieved approximately 0.5 hour post dose.
Distribution
The mean plasma protein binding of levocetirizine in vitro ranged from 91 to 92%, independent of concentration in the range of 90–5000 ng/mL, which includes the therapeutic plasma levels observed. Following oral dosing, the average apparent volume of distribution is approximately 0.4 L/kg, representative of distribution in total body water.
Metabolism
The extent of metabolism of levocetirizine in humans is less than 14% of the dose and therefore differences resulting from genetic polymorphism or concomitant intake of hepatic drug metabolizing enzyme inhibitors are expected to be negligible. Metabolic pathways include aromatic oxidation, N- and O-dealkylation, and taurine conjugation. Dealkylation pathways are primarily mediated by CYP3A4 while aromatic oxidation involves multiple and/or unidentified CYP isoforms.
Elimination
The plasma half-life in adult healthy subjects was about 8 to 9 hours after administration of oral tablets and oral solution, and the mean oral total body clearance for levocetirizine was approximately 0.63 mL/kg/min. The major route of excretion of levocetirizine and its metabolites is via urine, accounting for a mean of 85.4% of the dose. Excretion via feces accounts for only 12.9% of the dose. Levocetirizine is excreted both by glomerular filtration and active tubular secretion. Renal clearance of levocetirizine correlates with that of creatinine clearance. In patients with renal impairment the clearance of levocetirizine is reduced [see Dosage and Administration (2.2)].
Drug Interaction Studies
In vitro data on metabolite interaction indicate that levocetirizine is unlikely to produce, or be subject to metabolic interactions. Levocetirizine at concentrations well above Cmax level achieved within the therapeutic dose ranges is not an inhibitor of CYP isoenzymes 1A2, 2C9, 2C19, 2A1, 2D6, 2E1, and 3A4, and is not an inducer of UGT1A or CYP isoenzymes 1A2, 2C9 and 3A4.
No formal in vivo drug interaction studies have been performed with levocetirizine. Studies have been performed with the racemic cetirizine [see Drug Interactions (7)].
Pediatric patients
Data from a pediatric pharmacokinetic study with oral administration of a single dose of 5 mg levocetirizine in 14 children age 6 to 11 years with body weight ranging between 20 and 40 kg show that Cmax and AUC values are about 2-fold greater than that reported in healthy adult subjects in a cross-study comparison. The mean Cmax was 450 ng/mL, occurring at a mean time of 1.2 hours, weight-normalized, total body clearance was 30% greater, and the elimination half-life 24% shorter in this pediatric population than in adults.
Dedicated pharmacokinetic studies have not been conducted in pediatric patients younger than 6 years of age. A retrospective population pharmacokinetic analysis was conducted in 323 subjects (181 children 1 to 5 years of age, 18 children 6 to 11 years of age, and 124 adults 18 to 55 years of age) who received single or multiple doses of levocetirizine ranging from 1.25 mg to 30 mg. Data generated from this analysis indicated that administration of 1.25 mg once daily to children 6 months to 5 years of age results in plasma concentrations similar to those of adults receiving 5 mg once daily.
Geriatric patients
Limited pharmacokinetic data are available in elderly subjects. Following once daily repeat oral administration of 30 mg levocetirizine for 6 days in 9 elderly subjects (65–74 years of age), the total body clearance was approximately 33% lower compared to that in younger adults. The disposition of racemic cetirizine has been shown to be dependent on renal function rather than on age. This finding would also be applicable for levocetirizine, as levocetirizine and cetirizine are both predominantly excreted in urine. Therefore, the XYZAL dose should be adjusted in accordance with renal function in elderly patients [see Dosage and Administration (2)].
Gender
Pharmacokinetic results for 77 patients (40 men, 37 women) were evaluated for potential effect of gender. The half-life was slightly shorter in women (7.08 ± 1.72 hr) than in men (8.62 ± 1.84 hr); however, the body weight-adjusted oral clearance in women (0.67 ± 0.16 mL/min/kg) appears to be comparable to that in men (0.59 ± 0.12 mL/min/kg). The same daily doses and dosing intervals are applicable for men and women with normal renal function.
Race
The effect of race on levocetirizine has not been studied. As levocetirizine is primarily renally excreted, and there are no important racial differences in creatinine clearance, pharmacokinetic characteristics of levocetirizine are not expected to be different across races. No race-related differences in the kinetics of racemic cetirizine have been observed.
Renal impairment
Levocetirizine exposure (AUC) exhibited 1.8-, 3.2-, 4.3-, and 5.7-fold increase in mild, moderate, severe, renal impaired, and end-stage renal disease patients, respectively, compared to healthy subjects. The corresponding increases of half-life estimates were 1.4-, 2.0-, 2.9-, and 4-fold, respectively.
The total body clearance of levocetirizine after oral dosing was correlated to the creatinine clearance and was progressively reduced based on severity of renal impairment. Therefore, it is recommended to adjust the dose and dosing intervals of levocetirizine based on creatinine clearance in patients with mild, moderate, or severe renal impairment. In end-stage renal disease patients (CLCR < 10 mL/min) levocetirizine is contraindicated. The amount of levocetirizine removed during a standard 4-hour hemodialysis procedure was <10%.
The dosage of XYZAL should be reduced in patients with mild renal impairment. Both the dosage and frequency of administration should be reduced in patients with moderate or severe renal impairment [see Dosage and Administration (2.2)].
Hepatic impairment
XYZAL has not been studied in patients with hepatic impairment. The non-renal clearance (indicative of hepatic contribution) was found to constitute about 28% of the total body clearance in healthy adult subjects after oral administration.
As levocetirizine is mainly excreted unchanged by the kidney, it is unlikely that the clearance of levocetirizine is significantly decreased in patients with solely hepatic impairment [see Dosage and Administration (2)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been performed with levocetirizine. However, evaluation of cetirizine carcinogenicity studies is relevant for determination of the carcinogenic potential of levocetirizine. In a 2-year carcinogenicity study, in rats, cetirizine was not carcinogenic at dietary doses up to 20 mg/kg (approximately 40, 40, 25, and 10 times the MRHDs in adults, children 6 to 11 years of age, children 2–5 years, and children 6 months to 2 years of age, respectively, on a mg/m2 basis). In a 2-year carcinogenicity study in mice, cetirizine caused an increased incidence of benign hepatic tumors in males at a dietary dose of 16 mg/kg (approximately 15, 15, 9, and 5 times the MRHDs in adults, children 6 to 11 years of age, children 2–5 years, and children 6 months to 2 years of age, respectively, on a mg/m2 basis). No increased incidence of benign tumors was observed at a dietary dose of 4 mg/kg (approximately 4, 4, 2, and 1 times the MRHDs in adults, children 6 to 11 years of age, children 2–5 years, and children 6 months to 2 years of age, respectively on a mg/m2 basis). The clinical significance of these findings during long-term use of XYZAL is not known.
Levocetirizine was not mutagenic in the Ames test, and not clastogenic in the human lymphocyte assay, the mouse lymphoma assay, and in vivo micronucleus test in mice.
Fertility and reproductive performance were unaffected in male and female mice and rats that received cetirizine at oral doses up to 64 and 200 mg/kg/day, respectively (approximately 60 and 390 times the MRHD in adults on a mg/m2 basis).
-
14 CLINICAL STUDIES
14.1 Perennial Allergic Rhinitis
Adults and Adolescents 12 Years of Age and Older
The efficacy of XYZAL was evaluated in four randomized, placebo-controlled, double-blind clinical trials in adult and adolescent patients 12 years and older with symptoms of perennial allergic rhinitis. The four clinical trials include two dose-ranging trials of 4 weeks duration and two efficacy trials (one 6-week and one 6-month) in patients with perennial allergic rhinitis.
These trials included a total of 1729 patients (752 males and 977 females) of whom 227 were adolescents 12 to 17 years of age. Efficacy was assessed using a total symptom score from patient recording of 4 symptoms (sneezing, rhinorrhea, nasal pruritus, and ocular pruritus) in three studies and 5 symptoms (sneezing, rhinorrhea, nasal pruritus, ocular pruritus, and nasal congestion) in one study. Patients recorded symptoms using a 0–3 categorical severity scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe) once daily in the evening reflective of the 24 hour treatment period. The primary endpoint was the mean total symptom score averaged over the first week and over 4 weeks for perennial allergic rhinitis trials.
The two dose-ranging trials were conducted to evaluate the efficacy of XYZAL 2.5, 5, and 10 mg once daily in the evening. These trials were 4 weeks in duration and included patients with perennial allergic rhinitis. In these trials, each of the three doses of XYZAL demonstrated greater decrease in the reflective total symptom score than placebo and the difference was statistically significant for all three doses in the two studies. Results for one of these trials are shown in Table 4.
Table 4: Mean Reflective Total Symptom Score* in Allergic Rhinitis Dose-Ranging Trials Treatment N Baseline On Treatment Adjusted Mean Difference from Placebo Estimate 95% CI p-value - * Total symptom score is the sum of individual symptoms of sneezing, rhinorrhea, nasal pruritus, and ocular pruritus as assessed by patients on a 0–3 categorical severity scale.
Perennial Allergic Rhinitis Trial – Reflective total symptom score XYZAL 2.5 mg 133 7.14 4.12 1.17 (0.71, 1.63) <0.001 XYZAL 5 mg 127 7.18 4.07 1.22 (0.76, 1.69) <0.001 XYZAL 10 mg 129 7.58 4.19 1.10 (0.64, 1.57) <0.001 Placebo 128 7.22 5.29 One clinical trial evaluated the efficacy of XYZAL 5 mg once daily in the evening compared to placebo in patients with perennial allergic rhinitis over a 6-week treatment period. Another trial conducted over a 6-month treatment period assessed efficacy at 4 weeks. XYZAL 5 mg demonstrated a greater decrease from baseline in the reflective total symptom score than placebo and the difference from placebo was statistically significant. Results of the former are shown in Table 5.
Table 5: Mean Reflective Total Symptom Score* in Allergic Rhinitis Trials Treatment N Baseline On Treatment Adjusted Mean Difference from Placebo Estimate 95% CI p-value - * Total symptom score is the sum of individual symptoms of sneezing, rhinorrhea, nasal pruritus, and ocular pruritus as assessed by patients on a 0–3 categorical severity scale.
Perennial Allergic Rhinitis Trial – Reflective total symptom score XYZAL 5 mg 150 7.69 3.93 1.17 (0.70, 1.64) <0.001 Placebo 142 7.44 5.10 Onset of action was evaluated in two environmental exposure unit studies in allergic rhinitis patients with a single dose of XYZAL 2.5 or 5 mg. XYZAL 5 mg was found to have an onset of action 1 hour after oral intake. Onset of action was also assessed from the daily recording of symptoms in the evening before dosing in the allergic rhinitis trials. In these trials, onset of effect was seen after 1 day of dosing.
Pediatric Patients Less than 12 Years of Age
There are no clinical efficacy trials with XYZAL 2.5 mg once daily in pediatric patients under 12 years of age, and no clinical efficacy trials with XYZAL 1.25 mg once daily in pediatric patients 6 months to 5 years of age. The clinical efficacy of XYZAL in pediatric patients under 12 years of age has been extrapolated from adult clinical efficacy trials based on pharmacokinetic comparisons [see Use in Specific Populations (8.4)].
14.2 Chronic Idiopathic Urticaria
Adult Patients 18 Years of Age and Older
The efficacy of XYZAL for the treatment of the uncomplicated skin manifestations of chronic idiopathic urticaria was evaluated in two multi-center, randomized, placebo-controlled, double-blind clinical trials of 4 weeks duration in adult patients 18 to 85 years of age with chronic idiopathic urticaria. The two trials included one 4-week dose-ranging trial and one 4-week single-dose level efficacy trial. These trials included 423 patients (139 males and 284 females). Most patients (>90%) were Caucasian and the mean age was 41. Of these patients, 146 received XYZAL 5 mg once daily in the evening. Efficacy was assessed based on patient recording of pruritus severity on a severity score of 0–3 (0 = none to 3 = severe). The primary efficacy endpoint was the mean reflective pruritus severity score over the first week and over the entire treatment period. Additional efficacy variables were the instantaneous pruritus severity score, the number and size of wheals, and duration of pruritus.
The dose-ranging trial was conducted to evaluate the efficacy of XYZAL 2.5, 5, and 10 mg once daily in the evening. In this trial, each of the three doses of XYZAL demonstrated greater decrease in the reflective pruritus severity score than placebo and the difference was statistically significant for all three doses (see Table 6).
The single dose level trial evaluated the efficacy of XYZAL 5 mg once daily in the evening compared to placebo in patients with chronic idiopathic urticaria over a 4-week treatment period. XYZAL 5 mg demonstrated a greater decrease from baseline in the reflective pruritus severity score than placebo and the difference from placebo was statistically significant.
Duration of pruritus, number and size of wheals, and instantaneous pruritus severity score also showed significant improvement over placebo. The significant improvement in the instantaneous pruritus severity score over placebo confirmed end of dosing interval efficacy (see Table 6).
Table 6: Mean Reflective Pruritus Severity Score in Chronic Idiopathic Urticaria Trials Treatment N Baseline On Treatment Adjusted Mean Difference from Placebo Estimate 95% CI p-value Dose-Ranging Trial – Reflective pruritus severity score XYZAL 2.5 mg 69 2.08 1.02 0.82 (0.58, 1.06) <0.001 XYZAL 5 mg 62 2.07 0.92 0.91 (0.66, 1.16) <0.001 XYZAL 10 mg 55 2.04 0.73 1.11 (0.85, 1.37) <0.001 Placebo 60 2.25 1.84 Chronic Idiopathic Urticaria Trial – Reflective pruritus severity score XYZAL 5 mg 80 2.07 0.94 0.62 (0.38, 0.86) <0.001 Placebo 82 2.06 1.56 Pediatric Patients
There are no clinical efficacy trials in pediatric patients with chronic idiopathic urticaria [see Use in Specific Populations (8.4)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
XYZAL tablets are white, film-coated, oval-shaped, scored, imprinted (with the letter Y in red color on both halves of the scored tablet) and contain 5 mg levocetirizine dihydrochloride. They are supplied in unit of use HDPE bottles.
90 Tablets (NDC: 0024-5803-90)
XYZAL oral solution is a clear, colorless liquid containing 0.5 mg of levocetirizine dihydrochloride per mL.
Oral Solution in 5 oz polypropylene bottles (NDC: 0024-5804-05)
-
17 PATIENT COUNSELING INFORMATION
Somnolence
Caution patients against engaging in hazardous occupations requiring complete mental alertness, and motor coordination such as operating machinery or driving a motor vehicle after ingestion of XYZAL.
Concomitant Use of Alcohol and other Central Nervous System Depressants
Instruct patients to avoid concurrent use of XYZAL with alcohol or other central nervous system depressants because additional reduction in mental alertness may occur.
Dosing of XYZAL
Do not exceed the recommended daily dose in adults and adolescents 12 years of age and older of 5 mg once daily in the evening. In children 6 to 11 years of age the recommended dose is 2.5 mg once daily in the evening. In children 6 months to 5 years of age, the recommended dose is 1.25 mg once daily in the evening. Advise patients to not ingest more than the recommended dose of XYZAL because of the increased risk of somnolence at higher doses.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle Label
NDC: 0024-5803-90
Rx onlyXYZAL®
(levocetirizine
dihydrochloride)5 mg per tablet
For oral administration90 tablets
SANOFI

-
PRINCIPAL DISPLAY PANEL - 148 mL Bottle Carton
NDC: 0024-5804-05
Rx onlyXYZAL®
(levocetirizine dihydrochloride)
oral solution
2.5 mg/5 mL (0.5 mg/mL)5 oz (148 mL) bottle
SANOFI

-
INGREDIENTS AND APPEARANCE
XYZAL
levocetirizine dihydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0024-5803 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength levocetirizine dihydrochloride (UNII: SOD6A38AGA) (levocetirizine - UNII:6U5EA9RT2O) levocetirizine dihydrochloride 5 mg Inactive Ingredients Ingredient Name Strength microcrystalline cellulose (UNII: OP1R32D61U) lactose monohydrate (UNII: EWQ57Q8I5X) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) hypromellose, unspecified (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) polyethylene glycol 400 (UNII: B697894SGQ) FD&C Red No. 40 (UNII: WZB9127XOA) shellac (UNII: 46N107B71O) Product Characteristics Color WHITE Score 2 pieces Shape OVAL Size 8mm Flavor Imprint Code Y;Y Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0024-5803-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/24/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022064 07/24/2017 XYZAL
levocetirizine dihydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0024-5804 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength levocetirizine dihydrochloride (UNII: SOD6A38AGA) (levocetirizine - UNII:6U5EA9RT2O) levocetirizine dihydrochloride 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength sodium acetate (UNII: 4550K0SC9B) acetic acid (UNII: Q40Q9N063P) maltitol (UNII: D65DG142WK) glycerin (UNII: PDC6A3C0OX) methylparaben (UNII: A2I8C7HI9T) propylparaben (UNII: Z8IX2SC1OH) saccharin (UNII: FST467XS7D) triacetin (UNII: XHX3C3X673) .alpha.-tocopherol, dl- (UNII: 7QWA1RIO01) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0024-5804-05 1 in 1 CARTON 07/24/2017 1 148 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022157 07/24/2017 Labeler - Sanofi-Aventis U.S. LLC (824676584) Establishment Name Address ID/FEI Business Operations UCB Farchim S.A. 481797603 MANUFACTURE(0024-5803, 0024-5804) , API MANUFACTURE(0024-5803, 0024-5804) , ANALYSIS(0024-5803, 0024-5804) Establishment Name Address ID/FEI Business Operations Bausch Health Companies Inc. 245141858 MANUFACTURE(0024-5803) , ANALYSIS(0024-5803) , LABEL(0024-5803) , PACK(0024-5803)
Trademark Results [Xyzal]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 XYZAL 75874693 2479307 Live/Registered |
UCB BIOPHARMA SPRL 1999-12-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.