Sinus and Headache Day Night by GOODSENSE GDS-1149B-2020-0820
Sinus and Headache Day Night by
Drug Labeling and Warnings
Sinus and Headache Day Night by is a Otc medication manufactured, distributed, or labeled by GOODSENSE. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
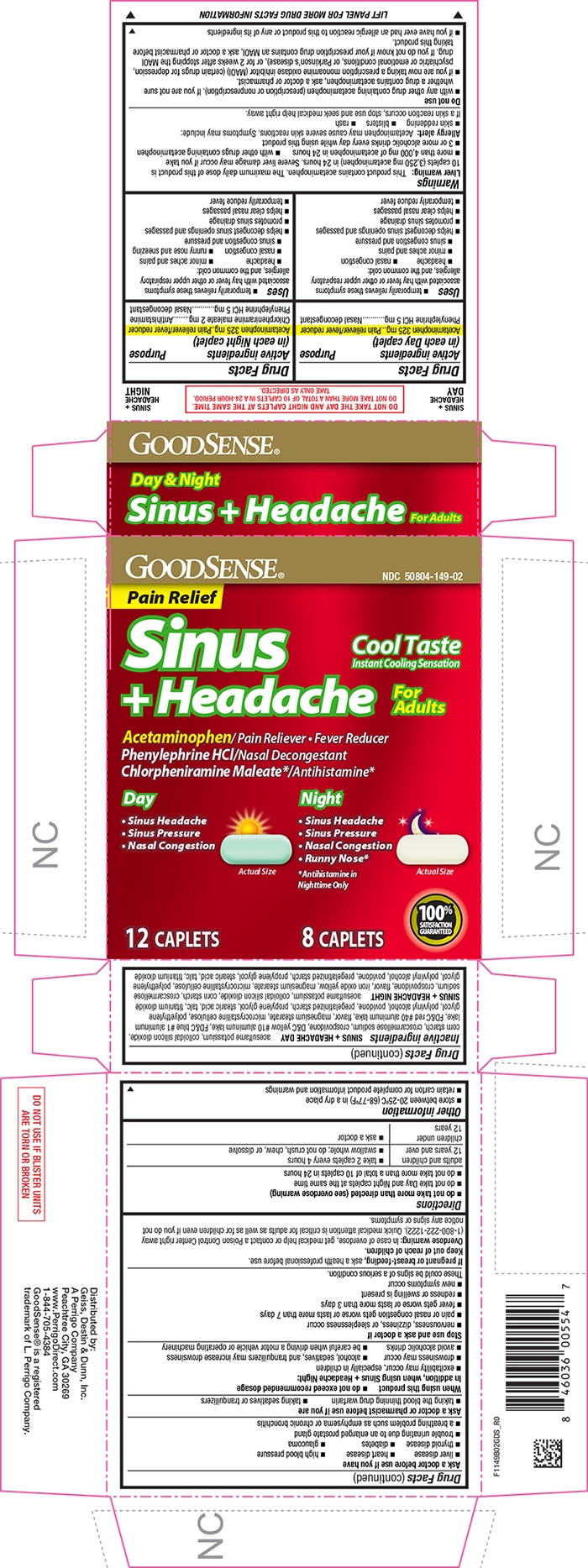
SINUS AND HEADACHE DAY NIGHT- acetaminophen, chlorpheniramine maleate, and phenylephrine hydrochloride
GOODSENSE
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
GDS-1149B-2020-0820
Uses
■ temporarily relieves these symptoms associated with hay fever or other upper respiratory allergies, and the common cold:
■ headache
■ sinus congestion and pressure
■ nasal congestion
■ minor aches and pains
■ helps decongest sinus openings and passages
■ promotes sinus drainage
■ helps clear nasal passages
■ temporarily reduces fever
Uses
■ temporarily relieves these symptoms associated with hay fever or other upper respiratory allergies, and the common cold:
■ headache
■ sinus congestion and pressure
■ nasal congestion
■ runny nose and sneezing
■ minor aches and pains
■ helps decongest sinus openings and passages
■ promotes sinus drainage
■ helps clear nasal passages
■ temporarily reduces fever
Warnings
Liver warning
Liver warning: This product contains acetaminophen. The maximum daily dose of this product is 10 caplets (3,250 mg acetaminophen) in 24 hours. Severe liver damage may occur if you take
■ more than 4,000 mg of acetaminophen in 24 hours
■ with other drugs containing acetaminophen
■ 3 or more alcoholic drinks every day while using this product
Allergy alert
Acetaminophen may cause severe skin reactions. Symptoms may include:
■ skin reddening
■ blisters
■ rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
■ if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
■ liver disease
■ heart disease
■ high blood pressure
■ thyroid disease
■ diabetes
■ trouble urinating due to an enlarged prostate gland
■ a breathing problem such as emphysema or chronic bronchitis
■ glaucoma
Ask a doctor or pharmacist before use if you are
■ taking the blood thinning drug warfarin
■ taking sedatives or tranquilizers
When using this product
■
do not exceed recommended dosage
In addition, when using Sinus + Headache Night:
■ excitability may occur, especially in children
■ drowsiness may occur
■ alcohol, sedatives, and tranquilizers may increase drowsiness
■ avoid alcoholic drinks
■ be careful when driving a motor vehicle or operating machinery
Directions
■ do not take more than directed (see overdose warning)
■ do not take Day and Night caplets at the same time
■ do not take more than a total of 10 caplets in 24 hours
| adults and children 12 years and over |
|
| children under 12 years |
|
Other information
■ store between 20-25ºC (68-77ºF) in a dry place
■ retain carton for complete product information and warnings
Inactive ingredients
SINUS + HEADACHE DAY
acesulfame potassium, colloidal silicon dioxide, croscarmellose sodium, crospovidone, D&C yellow #10 aluminum lake, FD&C blue #1 aluminum lake, FD&C red #40 aluminum lake, flavor, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, pregelatinized starch, propylene glycol, stearic acid, talc, titanium dioxide
PRINCIPAL DISPLAY PANEL
GOODSENSE®
NDC: 50804-149-02
Pain Relief
Sinus + Headache
Cool Taste Instant Cooling Sensation
For Adults
Acetaminophen/ Pain RelieverFever Reducer
Phenylephrine HCl/Nasal Decongestant
Chlorpheniramine Maleate*/Antihistamine*
Day
Sinus Headache
Sinus Pressure
Nasal Congestion
Actual Size
12 CAPLETS
Night
Sinus Headache
Sinus Pressure
Nasal Congestion
Runny Nose*
*antihistamine in Nighttime Only
Actual Size
8 CAPLETS
| SINUS AND HEADACHE DAY NIGHT
acetaminophen, chlorpheniramine maleate, and phenylephrine hydrochloride kit |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - GOODSENSE (076059836) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.