Stool Softener by Bene Health OTC / Geri-Care Pharmaceutical Corp ben 408
Stool Softener by
Drug Labeling and Warnings
Stool Softener by is a Otc medication manufactured, distributed, or labeled by Bene Health OTC, Geri-Care Pharmaceutical Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
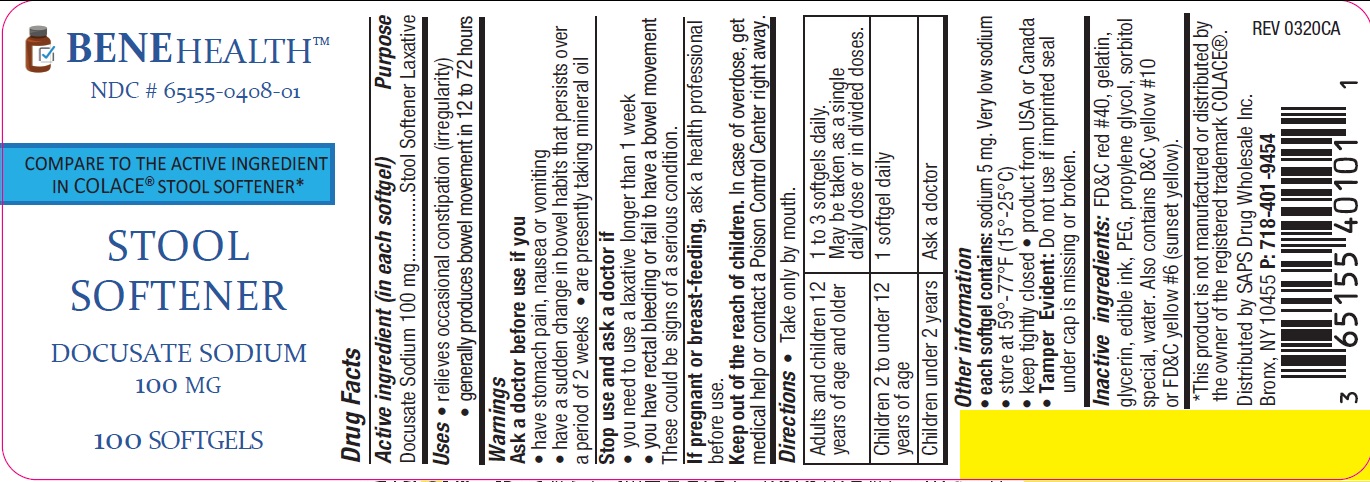
STOOL SOFTENER- docusate sodium capsule, liquid filled
Bene Health OTC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ben 408
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you
have stomach pain, nausea or vomiting
have a sudden change in bowel habits that persists over a period of 2 weeks
are presently taking mineral oil
Stop use and ask a doctor if
you need to use a laxative longer than 1 week
you have rectal bleeding or fail to have a bowel movement. These
could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Directions
Take only by mouth.
| Adults and children 12
years of age and older | 1 to 3 softgels daily.
May be taken as a single daily dose or in divided doses |
| Children 2 to under 12
years of age | 1 softgel daily |
| Children under 2 years | Ask a doctor |
Other information
each softgel contains: sodium 5 mg. very low sodium
store at 15°C-25°C(59° F-77° F)
keep tightly closed
product from USA or Canada
Tamper Evident: Do not use if imprinted seal under cap is missing or broken.
| STOOL SOFTENER
docusate sodium capsule, liquid filled |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Bene Health OTC (830110347) |
| Registrant - Geri-Care Pharmaceutical Corp (611196254) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Geri-Care Pharmaceutical Corp | 611196254 | pack(65155-408) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.