Intime Cesens by Nartex Laboratorios Homeopaticos SA DE CV
Intime Cesens by
Drug Labeling and Warnings
Intime Cesens by is a Homeopathic medication manufactured, distributed, or labeled by Nartex Laboratorios Homeopaticos SA DE CV. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
INTIME CESENS- intime cesens gel
Nartex Laboratorios Homeopaticos SA DE CV
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredient
Calendula officinalis TINC HPUS*
Chamomilla TINC HPUS*
Hamamelis virginiana 3x HPUS*
Hydrastis canadensis 5x HPUS*
*The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. "X" is an homeopathic dilution.
Purpose
Calendula officinalis TINC HPUS*......external vaginal burning
Chamomilla TINC HPUS*.....burning and vulvar itching
Hamamelis virginiana 3x HPUS*.....external vaginal irritation
Hydrastis canadensis 5x HPUS*.....itching and vulvar pruritis
USES
Temporary Relief of external vaginal
- burning
- itching
- irritation.
Claims based on traditional homeopathic practice, not accepted medical evidence. These uses have not been evaluated by FDA, and product has not been clinically tested.
Stop use and ask a doctor if
- symptoms persist for more than 7 days or clear up and occur again within a few days
- rash develops.
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults, cleanse the affected area with an appropriate cleansing wipe
- apply generously to affeted area up to 2 times daily
- reappy if needed
Other Information
- store at room temperature
- do not use if the tube seal is broken or missing. Claims based on traditional homeopathic practice, not accepted medical evidence. Theses uses have not been evaluated by FDA, and product has not been clinically tested.
Inactive Ingredients
Aloe Vera Leaf, citric acid, glycerin, lavander essential oil, potassium sorbate, propylene glycol, purified water, sodium benzoate, xanthan gum
Active Ingredients are prepared in accordance with the Homeopathic Pharmacopoeia of the United States, and are therefore non-toxic and have no known side effects. Claims based on traditional homeopathic practice, not accepted medical evidence. These uses have not been evaluated by FDA, and product has not been clnically tested.
Keep carton. It contains important information.
Product of Mexico / Producto de Mexico
Distributed by: Nartex Labs, USA, Inc.
9543 Bissonnet St. STE. 302
Houston, TX 77036 USA
www.nartexlabsusa.com
PRINCIPAL DISPLAY PANEL
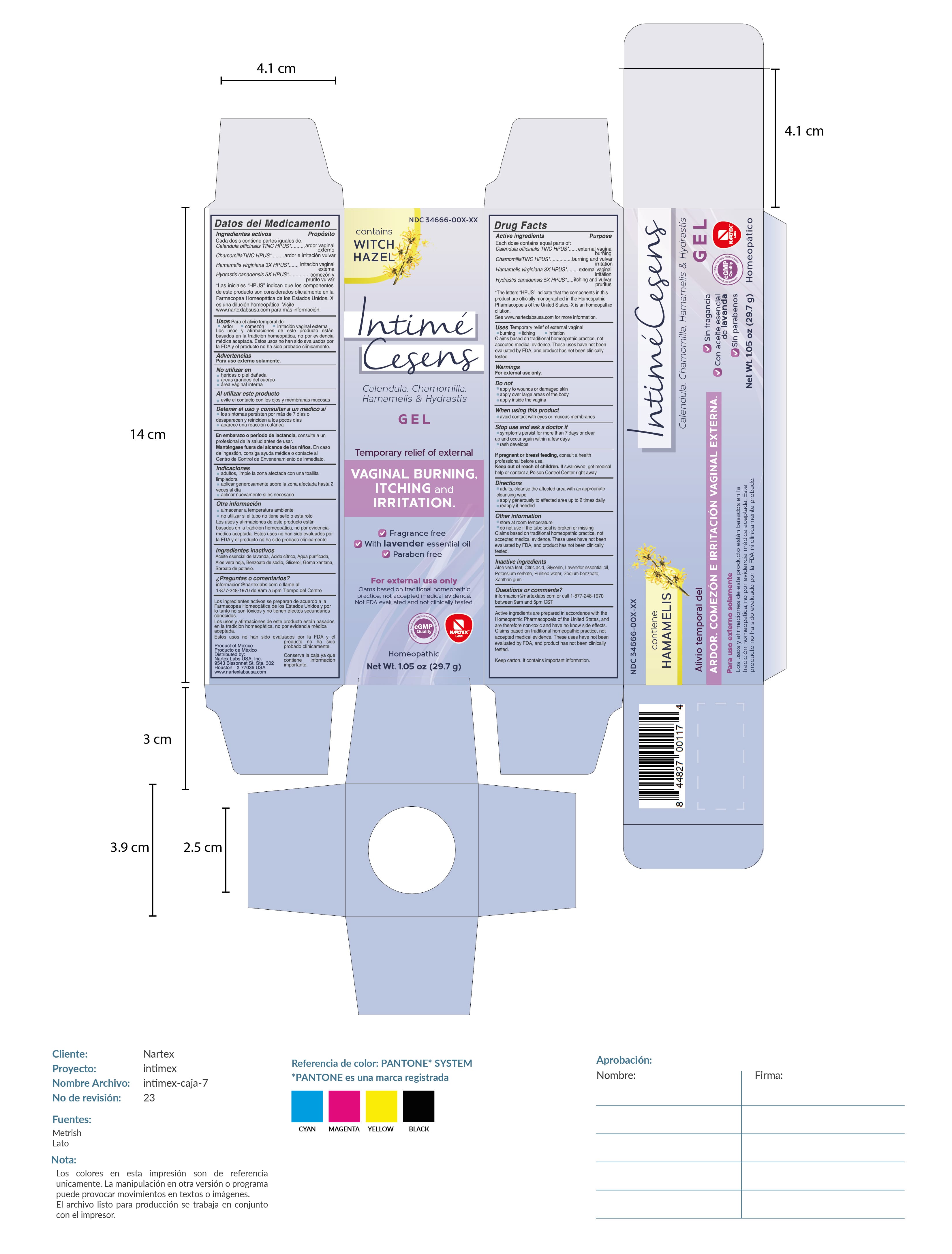 Contains witch hazel
Contains witch hazel
Intime Cesens
Calendula, Chamomila, Hamamelis & Hydrastis Gel
Temporary relief of external vaginal burning, itching and irritation.
fragrance free. with lavender essential oil. paraben free
For external use only
Claims based on traditional homeopathic practice, not acceptd medical evidence. Not FDA evaluated and not clinically tested.
Homeopathic
Net Wt. 1.05oz (29.7g)
| INTIME CESENS
intime cesens gel |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Nartex Laboratorios Homeopaticos SA DE CV (589914576) |
| Registrant - Nartex Laboratorios Homeopaticos SA DE CV (589914576) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nartex Laboratorios Homeopaticos SA DE CV | 589914576 | manufacture(34666-301) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.