COLCRYS- colchicine tablet, film coated
Colcrys by
Drug Labeling and Warnings
Colcrys by is a Prescription medication manufactured, distributed, or labeled by Aphena Pharma Solutions - Tennessee, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use colchicine safely and effectively. See full prescribing information for COLCRYS®.
COLCRYS® (colchicine, USP) tablets for Oral use
Initial U.S. Approval: 1961INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Gout Flares:
- FMF: Adults and Children older than 12 years 1.2 – 2.4 mg; Children 6 to 12 years 0.9 – 1.8 mg; Children 4 to 6 years 0.3 – 1.8 mg (2.2, 2.3).
Colchicine tablets are administered orally, without regard to meals.
See full prescribing information for dose adjustment regarding patients with impaired renal function (2.5), impaired hepatic function (2.6), the patient's age (2.3, 8.5), or use of co-administered drugs (2.4).
DOSAGE FORMS AND STRENGTHS
- 0.6 mg tablets (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Fatal overdoses have been reported with colchicine in adults and children. Keep COLCRYS out of the reach of children (5.1, 10).
- Blood dyscrasias: myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, and aplastic anemia have been reported (5.2).
- Monitor for toxicity and if present consider temporary interruption or discontinuation of colchicine (5.2, 5.3, 5.4, 6, 10).
- Drug interaction P-gp and/or CYP3A4 inhibitors: Co-administration of colchicine with P-gp and/or strong CYP3A4 inhibitors has resulted in life-threatening interactions and death (5.3, 7).
- Neuromuscular toxicity: Myotoxicity including rhabdomyolysis may occur, especially in combination with other drugs known to cause this effect. Consider temporary interruption or discontinuation of COLCRYS (5.4, 7).
ADVERSE REACTIONS
Prophylaxis of Gout Flares: The most commonly reported adverse reaction in clinical trials for the prophylaxis of gout was diarrhea.
Treatment of Gout Flares: The most common adverse reactions reported in the clinical trial for gout were diarrhea (23%) and pharyngolaryngeal pain (3%).
FMF: Most common adverse reactions (up to 20%) are abdominal pain, diarrhea, nausea, and vomiting. These effects are usually mild, transient, and reversible upon lowering the dose (6).
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals America, Inc. at 1-877-825-3327 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Co-administration of P-gp and/or CYP3A4 inhibitors (e.g., clarithromycin or cyclosporine) have been demonstrated to alter the concentration of colchicine. The potential for drug-drug interactions must be considered prior to and during therapy. See full prescribing information for a complete list of reported and potential interactions (2.4, 5.3, 7).
USE IN SPECIFIC POPULATIONS
- In the presence of mild to moderate renal or hepatic impairment, adjustment of dosing is not required for treatment of gout flare, prophylaxis of gout flare, and FMF but patients should be monitored closely (2.5, 8.6).
- In patients with severe renal impairment for prophylaxis of gout flares the starting dose should be 0.3 mg/day, for gout flares no dose adjustment is required but a treatment course should be repeated no more than once every 2 weeks. In FMF patients, start with 0.3 mg/day and any increase in dose should be done with close monitoring (2.5, 8.6).
- In patients with severe hepatic impairment, a dose reduction may be needed in prophylaxis of gout flares and FMF patients; while a dose reduction may not be needed in gout flares, a treatment course should be repeated no more than once every 2 weeks (2.5, 2.6, 8.6, 8.7).
- For patients undergoing dialysis, the total recommended dose for prophylaxis of gout flares should be 0.3 mg given twice a week with close monitoring. For treatment of gout flares, the total recommended dose should be reduced to 0.6 mg (1 tablet) × 1 dose and the treatment course should not be repeated more than once every two weeks. For FMF patients the starting dose should be 0.3 mg per day and dosing can be increased with close monitoring (2.5, 8.6).
- Pregnancy: Use only if the potential benefit justifies the potential risk to the fetus (8.1).
- Nursing Mothers: Caution should be exercised when administered to a nursing woman (8.3).
- Geriatric Use: The recommended dose of colchicine should be based on renal function (2.5, 8.5).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2014
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Gout Flares
1.2 Familial Mediterranean fever (FMF)
2 DOSAGE AND ADMINISTRATION
2.1 Gout Flares
2.2 FMF
2.3 Recommended Pediatric Dosage
2.4 Dose Modification for Co-administration of Interacting Drugs
2.5 Dose Modification in Renal Impairment
2.6 Dose Modification in Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fatal Overdose
5.2 Blood Dyscrasias
5.3 Drug Interactions
5.4 Neuromuscular Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience in Gout
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
17.1 Dosing Instructions
17.2 Fatal Overdose
17.3 Blood Dyscrasias
17.4 Drug and Food Interactions
17.5 Neuromuscular Toxicity
17.6 Medication Guide
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Gout Flares
COLCRYS® (colchicine, USP) tablets are indicated for prophylaxis and the treatment of acute gout flares.
-
Prophylaxis of Gout Flares:
COLCRYS is indicated for prophylaxis of gout flares. -
Treatment of Gout Flares:
COLCRYS tablets are indicated for treatment of acute gout flares when taken at the first sign of a flare.
-
Prophylaxis of Gout Flares:
-
2 DOSAGE AND ADMINISTRATION
The long term use of colchicine is established for FMF and the prophylaxis of gout flares but the safety and efficacy of repeat treatment for gout flares has not been evaluated. The dosing regimens for COLCRYS are different for each indication and must be individualized.
The recommended dosage of COLCRYS depends on the patient's age, renal function, hepatic function, and use of co-administered drugs [see Dose Modification for Co-administration of Interacting Drugs (2.4)].
COLCRYS tablets are administered orally, without regard to meals.
COLCRYS is not an analgesic medication and should not be used to treat pain from other causes.
2.1 Gout Flares
Prophylaxis of Gout Flares:
The recommended dosage of COLCRYS for prophylaxis of gout flares for adults and adolescents older than 16 years of age is 0.6 mg once or twice daily. The maximum recommended dose for prophylaxis of gout flares is 1.2 mg/day.
Treatment of Gout Flares:
The recommended dose of COLCRYS for treatment of a gout flare is 1.2 mg (2 tablets) at the first sign of the flare followed by 0.6 mg (1 tablet) one hour later. Higher doses have not been found to be more effective. The maximum recommended dose for treatment of gout flares is 1.8 mg over a 1 hour period. COLCRYS may be administered for treatment of a gout flare during prophylaxis at doses not to exceed 1.2 mg (2 tablets) at the first sign of the flare followed by 0.6 mg (1 tablet) one hour later. Wait 12 hours and then resume the prophylactic dose.
2.2 FMF
The recommended dosage of COLCRYS for FMF in adults is 1.2 mg to 2.4 mg daily.
COLCRYS should be increased as needed to control disease and as tolerated in increments of 0.3 mg/day to a maximum recommended daily dose. If intolerable side effects develop, the dose should be decreased in increments of 0.3 mg/day. The total daily COLCRYS dose may be administered in one to two divided doses.
2.3 Recommended Pediatric Dosage
Prophylaxis and Treatment of Gout Flares:
COLCRYS is not recommended for pediatric use in prophylaxis or treatment of gout flares.
FMF:
The recommended dosage of COLCRYS for FMF in pediatric patients 4 years of age and older is based on age. The following daily doses may be given as a single or divided dose twice daily:
- Children 4 – 6 years: 0.3 mg to 1.8 mg daily
- Children 6 – 12 years: 0.9 mg to 1.8 mg daily
- Adolescents older than 12 years: 1.2 mg to 2.4 mg daily
2.4 Dose Modification for Co-administration of Interacting Drugs
Concomitant Therapy:
Co-administration of COLCRYS with drugs known to inhibit CYP3A4 and/or P-glycoprotein (P-gp) increases the risk of colchicine-induced toxic effects (Table 1). If patients are taking or have recently completed treatment with drugs listed in Table 1 within the prior 14 days, the dose adjustments are as shown on the table below [see DRUG INTERACTIONS (7)].
Table 1 COLCRYS Dose Adjustment for Co-administration with Interacting Drugs if no Alternative Available* - * For magnitude of effect on colchicine plasma concentrations [see Pharmacokinetics (12.3)]
- † Patients with renal or hepatic impairment should not be given COLCRYS in conjunction with strong CYP3A4 or P-gp inhibitors [see CONTRAINDICATIONS (4)].
- ‡ When used in combination with Ritonavir, see dosing recommendations for strong CYP3A4 inhibitors [see CONTRAINDICATIONS (4)].
Strong CYP3A4 Inhibitors†
Drug
Noted or Anticipated Outcome
Gout Flares
FMF
Prophylaxis of Gout Flares
Treatment of Gout Flares
Original Intended Dosage
Adjusted Dose
Original Intended Dosage
Adjusted Dose
Original Intended Dosage
Adjusted Dose
Atazanavir
Clarithromycin
Darunavir/
Ritonavir‡
Indinavir
Itraconazole Ketoconazole
Lopinavir/
Ritonavir‡
Nefazodone
Nelfinavir
Ritonavir
Saquinavir Telithromycin
Tipranavir/
Ritonavir‡Significant increase in colchicine plasma levels*; fatal colchicine toxicity has been reported with clarithromycin, a strong CYP3A4 inhibitor. Similarly, significant increase in colchicine plasma levels is anticipated with other strong CYP3A4 inhibitors.
0.6 mg twice a day
0.6 mg once a day0.3 mg once a day
0.3 mg once every other day1.2 mg
(2 tablets) followed by 0.6 mg (1 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.0.6 mg
(1 tablet) × 1 dose, followed by 0.3 mg (1/2 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.Maximum daily dose of 1.2 – 2.4 mg
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day)
Moderate CYP3A4 Inhibitors
Drug
Noted or Anticipated Outcome
Gout Flares
FMF
Prophylaxis of Gout Flares
Treatment of Gout Flares
Original Intended Dosage
Adjusted Dose
Original Intended Dosage
Adjusted Dose
Original Intended Dosage
Adjusted Dose
Amprenavir‡ Aprepitant
Diltiazem Erythromycin Fluconazole Fosamprenavir‡
(pro-drug of
Amprenavir)
Grapefruit Juice
VerapamilSignificant increase in colchicine plasma concentration is anticipated. Neuromuscular toxicity has been reported with diltiazem and verapamil interactions.
0.6 mg twice a day
0.6 mg once a day0.3 mg twice a day or 0.6 mg once a day
0.3 mg once a day1.2 mg
(2 tablets) followed by 0.6 mg (1 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.1.2 mg
(2 tablets) × 1 dose. Dose to be repeated no earlier than 3 days.Maximum daily dose of 1.2 – 2.4 mg.
Maximum daily dose of 1.2 mg (may be given as 0.6 mg twice a day)
P-gp Inhibitors†
Drug
Noted or Anticipated Outcome
Gout Flares
FMF
Prophylaxis of Gout Flares
Treatment of Gout Flares
Original Intended Dosage
Adjusted Dose
Original Intended Dosage
Adjusted Dose
Original Intended Dosage
Adjusted Dose
Cyclosporine Ranolazine
Significant increase in colchicine plasma levels*; fatal colchicine toxicity has been reported with cyclosporine, a P-gp inhibitor. Similarly, significant increase in colchicine plasma levels is anticipated with other P-gp inhibitors.
0.6 mg twice a day
0.6 mg once a day
0.3 mg once a day
0.3 mg once every other day1.2 mg
(2 tablets) followed by 0.6 mg (1 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.0.6 mg
(1 tablet) × 1 dose. Dose to be repeated no earlier than 3 days.Maximum daily dose of 1.2 – 2.4 mg
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day)
Table 2 COLCRYS Dose Adjustment for Co-administration with Protease Inhibitors Protease Inhibitor
Clinical Comment
w/Colchicine – Prophylaxis of Gout Flares
w/Colchicine –
Treatment of Gout Flaresw/Colchicine – Treatment of FMF
Atazanavir sulfate
(Reyataz)Patients with renal or hepatic impairment should not be given colchicine with Reyataz.
Original dose
Adjusted dose
0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (1/2 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day)
0.6 mg twice a day
0.6 mg once a day0.3 mg once a day
0.3 mg once every other dayDarunavir (Prezista)
Patients with renal or hepatic impairment should not be given colchicine with Prezista/ritonavir.
Original dose
Adjusted dose
0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (1/2 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day)
0.6 mg twice a day
0.6 mg once a day0.3 mg once a day
0.3 mg once every other dayFosamprenavir (Lexiva) with Ritonavir
Patients with renal or hepatic impairment should not be given colchicine with Lexiva/ritonavir.
Original dose
Adjusted dose
0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (1/2 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day)
0.6 mg twice a day
0.6 mg once a day0.3 mg once a day
0.3 mg once every other dayFosamprenavir (Lexiva)
Patients with renal or hepatic impairment should not be given colchicine with Lexiva/ritonavir.
Original dose
Adjusted dose
1.2 mg (2 tablets) × 1 dose. Dose to be repeated no earlier than 3 days.
Maximum daily dose of 1.2 mg (may be given as 0.6 mg twice a day)
0.6 mg twice a day
0.6 mg once a day0.3 mg twice a day or 0.6 mg once a day
0.3 mg once a dayIndinavir (Crixivan)
Patients with renal or hepatic impairment should not be given colchicine with Crixivan.
Original dose
Adjusted dose
0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (1/2 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day)
0.6 mg twice a day
0.6 mg once a day0.3 mg once a day
0.3 mg once every other dayLopinavir/Ritonavir (Kaletra)
Patients with renal or hepatic impairment should not be given colchicine with Kaletra.
Original dose
Adjusted dose
0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (1/2 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day)
0.6 mg twice a day
0.6 mg once a day0.3 mg once a day
0.3 mg once every other dayNelfinavir mesylate (Viracept)
Patients with renal or hepatic impairment should not be given colchicine with Viracept.
Original dose
Adjusted dose
0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (1/2 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day)
0.6 mg twice a day
0.6 mg once a day0.3 mg once a day
0.3 mg once every other dayRitonavir (Norvir)
Patients with renal or hepatic impairment should not be given colchicine with Norvir.
Original dose
Adjusted dose
0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (1/2 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day)
0.6 mg twice a day
0.6 mg once a day0.3 mg once a day
0.3 mg once every other daySaquinavir mesylate (Invirase)
Patients with renal or hepatic impairment should not be given colchicine with Invirase/ritonavir.
Original dose
Adjusted dose
0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (1/2 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day)
0.6 mg twice a day
0.6 mg once a day0.3 mg once a day
0.3 mg once every other dayTipranavir (Aptivus)
Patients with renal or hepatic impairment should not be given colchicine with Aptivus/ritonavir.
Original dose
Adjusted dose
0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (1/2 tablet) 1 hour later. Dose to be repeated no earlier than 3 days.
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day)
0.6 mg twice a day
0.6 mg once a day0.3 mg once a day
0.3 mg once every other dayTreatment of gout flares with COLCRYS is not recommended in patients receiving prophylactic dose of COLCRYS and CYP3A4 inhibitors.
2.5 Dose Modification in Renal Impairment
Colchicine dosing must be individualized according to the patient's renal function [see Renal Impairment (8.6)].
Clcr in mL/minute may be estimated from serum creatinine (mg/dL) determination using the following formula:
Clcr =
[140-age (years) × weight (kg)]
× 0.85 for female patients
72 × serum creatinine (mg/dL)
Gout Flares:
Prophylaxis of Gout Flares:
For prophylaxis of gout flares in patients with mild (estimated creatinine clearance Clcr 50 – 80 mL/min) to moderate (Clcr 30 – 50 mL/min) renal function impairment, adjustment of the recommended dose is not required, but patients should be monitored closely for adverse effects of colchicine. However, in patients with severe impairment, the starting dose should be 0.3 mg per day and any increase in dose should be done with close monitoring. For the prophylaxis of gout flares in patients undergoing dialysis, the starting doses should be 0.3 mg given twice a week with close monitoring [see Clinical Pharmacology (12.3) and Renal Impairment (8.6)].
Treatment of Gout Flares:
For treatment of gout flares in patients with mild (Clcr 50 – 80 mL/min) to moderate (Clcr 30 – 50 mL/min) renal function impairment, adjustment of the recommended dose is not required, but patients should be monitored closely for adverse effects of colchicine. However, in patients with severe impairment, while the dose does not need to be adjusted for the treatment of gout flares, a treatment course should be repeated no more than once every 2 weeks. For patients with gout flares requiring repeated courses consideration should be given to alternate therapy. For patients undergoing dialysis, the total recommended dose for the treatment of gout flares should be reduced to a single dose of 0.6 mg (1 tablet). For these patients, the treatment course should not be repeated more than once every 2 weeks [see Clinical Pharmacology (12.3) and Renal Impairment (8.6)].
Treatment of gout flares with COLCRYS is not recommended in patients with renal impairment who are receiving COLCRYS for prophylaxis.
FMF:
Caution should be taken in dosing patients with moderate and severe renal impairment and in patients undergoing dialysis. For these patients, the dosage should be reduced [see Clinical Pharmacology (12.3)]. Patients with mild (Clcr 50 – 80 mL/min) and moderate (Clcr 30 – 50 mL/min) renal impairment should be monitored closely for adverse effects of COLCRYS. Dose reduction may be necessary. For patients with severe renal failure (Clcr less than 30 mL/minute), start with 0.3 mg/day; any increase in dose should be done with adequate monitoring of the patient for adverse effects of colchicine [see Renal Impairment (8.6)]. For patients undergoing dialysis, the total recommended starting dose should be 0.3 mg (half tablet) per day. Dosing can be increased with close monitoring. Any increase in dose should be done with adequate monitoring of the patient for adverse effects of colchicine [see Clinical Pharmacology (12.3) and Renal Impairment (8.6)].
2.6 Dose Modification in Hepatic Impairment
Gout Flares
Prophylaxis of Gout Flares:
For prophylaxis of gout flares in patients with mild to moderate hepatic function impairment, adjustment of the recommended dose is not required, but patients should be monitored closely for adverse effects of colchicine. Dose reduction should be considered for the prophylaxis of gout flares in patients with severe hepatic impairment [see Hepatic Impairment (8.7)].
Treatment of Gout Flares:
For treatment of gout flares in patients with mild to moderate hepatic function impairment, adjustment of the recommended dose is not required, but patients should be monitored closely for adverse effects of colchicine. However, for the treatment of gout flares in patients with severe impairment while the dose does not need to be adjusted, but a treatment course should be repeated no more than once every 2 weeks. For these patients, requiring repeated courses for the treatment of gout flares, consideration should be given to alternate therapy [see Hepatic Impairment (8.7)].
Treatment of gout flares with COLCRYS is not recommended in patients with hepatic impairment who are receiving COLCRYS for prophylaxis.
FMF:
Patients with mild to moderate hepatic impairment should be monitored closely for adverse effects of colchicine. Dose reduction should be considered in patients with severe hepatic impairment [see Hepatic Impairment (8.7)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Patients with renal or hepatic impairment should not be given COLCRYS in conjunction with P-gp or strong CYP3A4 inhibitors (this includes all protease inhibitors, except fosamprenavir). In these patients, life-threatening and fatal colchicine toxicity has been reported with colchicine taken in therapeutic doses.
-
5 WARNINGS AND PRECAUTIONS
5.1 Fatal Overdose
Fatal overdoses, both accidental and intentional, have been reported in adults and children who have ingested colchicine [see OVERDOSAGE (10)]. COLCRYS should be kept out of the reach of children.
5.2 Blood Dyscrasias
Myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, pancytopenia, and aplastic anemia have been reported with colchicine used in therapeutic doses.
5.3 Drug Interactions
Colchicine is a P-gp and CYP3A4 substrate. Life-threatening and fatal drug interactions have been reported in patients treated with colchicine given with P-gp and strong CYP3A4 inhibitors. If treatment with a P-gp or strong CYP3A4 inhibitor is required in patients with normal renal and hepatic function, the patient's dose of colchicine may need to be reduced or interrupted [see DRUG INTERACTIONS (7)]. Use of COLCRYS in conjunction with P-gp or strong CYP3A4 inhibitors (this includes all protease inhibitors, except fosamprenavir) is contraindicated in patients with renal or hepatic impairment [see CONTRAINDICATIONS (4)].
5.4 Neuromuscular Toxicity
Colchicine-induced neuromuscular toxicity and rhabdomyolysis have been reported with chronic treatment in therapeutic doses. Patients with renal dysfunction and elderly patients, even those with normal renal and hepatic function, are at increased risk. Concomitant use of atorvastatin, simvastatin, pravastatin, fluvastatin, lovastatin, gemfibrozil, fenofibrate, fenofibric acid, or benzafibrate (themselves associated with myotoxicity) or cyclosporine with COLCRYS may potentiate the development of myopathy [see DRUG INTERACTIONS (7)]. Once colchicine is stopped, the symptoms generally resolve within 1 week to several months.
-
6 ADVERSE REACTIONS
Prophylaxis of Gout Flares:
The most commonly reported adverse reaction in clinical trials of colchicine for the prophylaxis of gout was diarrhea.
Treatment of Gout Flares:
The most common adverse reactions reported in the clinical trial with COLCRYS for treatment of gout flares were diarrhea (23%) and pharyngolaryngeal pain (3%).
FMF:
Gastrointestinal tract adverse effects are the most frequent side effects in patients initiating COLCRYS, usually presenting within 24 hours, and occurring in up to 20% of patients given therapeutic doses. Typical symptoms include cramping, nausea, diarrhea, abdominal pain, and vomiting. These events should be viewed as dose-limiting if severe as they can herald the onset of more significant toxicity.
6.1 Clinical Trials Experience in Gout
Because clinical studies are conducted under widely varying and controlled conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug, and may not predict the rates observed in a broader patient population in clinical practice.
In a randomized, double-blind, placebo-controlled trial in patients with a gout flare, gastrointestinal adverse reactions occurred in 26% of patients using the recommended dose (1.8 mg over 1 hour) of COLCRYS compared to 77% of patients taking a non-recommended high-dose (4.8 mg over 6 hours) of colchicine and 20% of patients taking placebo. Diarrhea was the most commonly reported drug-related gastrointestinal adverse event. As shown in Table 3, diarrhea is associated with COLCRYS treatment. Diarrhea was more likely to occur in patients taking the high-dose regimen than the low-dose regimen. Severe diarrhea occurred in 19% and vomiting occurred in 17% of patients taking the non-recommended high-dose colchicine regimen but did not occur in the recommended low-dose COLCRYS regimen.
Table 3 Number (%) of Patients with at Least One Drug-Related Treatment Emergent Adverse Events with an Incidence of ≥ 2% of Patients in Any Treatment Group MedDRA System Organ Class
MedDRA Preferred Term
COLCRYS Dose
Placebo
(N=59)
n (%)High (N=52)
n (%)Low (N=74)
n (%)Number of Patients with at Least One Drug-Related TEAE
40 (77)
27 (37)
16 (27)
Gastrointestinal Disorders
40 (77)
19 (26)
12 (20)
Diarrhea
40 (77)
17 (23)
8 (14)
Nausea
9 (17)
3 (4)
3 (5)
Vomiting
9 (17)
0
0
Abdominal Discomfort
0
0
2 (3)
General Disorders and Administration Site Conditions
4 (8)
1 (1)
1 (2)
Fatigue
2 (4)
1 (1)
1 (2)
Metabolic and Nutrition Disorders
0
3 (4)
2 (3)
Gout
0
3 (4)
1 (2)
Nervous System Disorders
1 (2)
1 (1.4)
2 (3)
Headache
1 (2)
1 (1)
2 (3)
Respiratory Thoracic Mediastinal Disorders
1 (2)
2 (3)
0
Pharyngolaryngeal Pain
1 (2)
2 (3)
0
6.2 Postmarketing Experience
Serious toxic manifestations associated with colchicine include myelosuppression, disseminated intravascular coagulation, and injury to cells in the renal, hepatic, circulatory, and central nervous systems.
These most often occur with excessive accumulation or overdosage [see OVERDOSAGE (10)].
The following adverse reactions have been reported with colchicine. These have been generally reversible upon temporarily interrupting treatment or lowering the dose of colchicine.
- Neurological: sensory motor neuropathy
- Dermatological: alopecia, maculopapular rash, purpura, rash
- Digestive: abdominal cramping, abdominal pain, diarrhea, lactose intolerance, nausea, vomiting
- Hematological: leukopenia, granulocytopenia, thrombocytopenia, pancytopenia, aplastic anemia
- Hepatobiliary: elevated AST, elevated ALT
- Musculoskeletal: myopathy, elevated CPK, myotonia, muscle weakness, muscle pain, rhabdomyolysis
- Reproductive: azoospermia, oligospermia
-
7 DRUG INTERACTIONS
COLCRYS (colchicine) is a substrate of the efflux transporter P-glycoprotein (P-gp). Of the cytochrome P450 enzymes tested, CYP3A4 was mainly involved in the metabolism of colchicine. If COLCRYS is administered with drugs that inhibit P-gp, most of which also inhibit CYP3A4, increased concentrations of colchicine are likely. Fatal drug interactions have been reported.
Physicians should ensure that patients are suitable candidates for treatment with COLCRYS and remain alert for signs and symptoms of toxicities related to increased colchicine exposure as a result of a drug interaction. Signs and symptoms of COLCRYS toxicity should be evaluated promptly and, if toxicity is suspected, COLCRYS should be discontinued immediately.
Table 4 provides recommendations as a result of other potentially significant drug interactions. Table 1 provides recommendations for strong and moderate CYP3A4 inhibitors and P-gp inhibitors.
Table 4 Other Potentially Significant Drug Interactions Concomitant Drug Class or Food
Noted or anticipated Outcome
Clinical Comment
HMG-Co A Reductase Inhibitors:
atorvastatin, fluvastatin, lovastatin, pravastatin, simvastatinPharmacokinetic and/or pharmacodynamic interaction: the addition of one drug to a stable long-term regimen of the other has resulted in myopathy and rhabdomyolysis (including a fatality)
Weigh the potential benefits and risks and carefully monitor patients for any signs or symptoms of muscle pain, tenderness, or weakness, particularly during initial therapy; monitoring CPK (creatine phosphokinase) will not necessarily prevent the occurrence of severe myopathy.
Other Lipid Lowering Drugs:
fibrates, gemfibrozilDigitalis Glycosides:
digoxinP-gp substrate; rhabdomyolysis has been reported
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies with colchicine in pregnant women. Colchicine crosses the human placenta. While not studied in the treatment of gout flares, data from a limited number of published studies found no evidence of an increased risk of miscarriage, stillbirth, or teratogenic effects among pregnant women using colchicine to treat familial Mediterranean fever (FMF). Although animal reproductive and developmental studies were not conducted with COLCRYS, published animal reproduction and development studies indicate that colchicine causes embryofetal toxicity, teratogenicity, and altered postnatal development at exposures within or above the clinical therapeutic range. COLCRYS should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Colchicine is excreted into human milk. Limited information suggests that exclusively breast-fed infants receive less than 10 percent of the maternal weight-adjusted dose. While there are no published reports of adverse effects in breast-feeding infants of mothers taking colchicine, colchicine can affect gastrointestinal cell renewal and permeability. Caution should be exercised and breast-feeding infants should be observed for adverse effects when COLCRYS is administered to a nursing woman.
8.4 Pediatric Use
The safety and efficacy of colchicine in children of all ages with FMF has been evaluated in uncontrolled studies. There does not appear to be an adverse effect on growth in children with FMF treated long-term with colchicine. Gout is rare in pediatric patients, safety and effectiveness of colchicine in pediatric patients has not been established.
8.5 Geriatric Use
Clinical studies with colchicine for prophylaxis and treatment of gout flares and for treatment of FMF did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient with gout should be cautious, reflecting the greater frequency of decreased renal function, concomitant disease, or other drug therapy [see Dose Modification for Co-administration of Interacting Drugs (2.4)].
8.6 Renal Impairment
Colchicine is significantly excreted in urine in healthy subjects. Clearance of colchicine is decreased in patients with impaired renal function. Total body clearance of colchicine was reduced by 75% in patients with end-stage renal disease undergoing dialysis.
Prophylaxis of Gout Flares:
For prophylaxis of gout flares in patients with mild (estimated creatinine clearance Clcr 50 – 80 mL/min) to moderate (Clcr 30 – 50 mL/min) renal function impairment, adjustment of the recommended dose is not required, but patients should be monitored closely for adverse effects of colchicine. However, in patients with severe impairment, the starting dose should be 0.3 mg per day and any increase in dose should be done with close monitoring. For the prophylaxis of gout flares in patients undergoing dialysis, the starting doses should be 0.3 mg given twice a week with close monitoring [see Dose Modification in Renal Impairment (2.5)].
Treatment of Gout Flares:
For treatment of gout flares in patients with mild (Clcr 50 – 80 mL/min) to moderate (Clcr 30 – 50 mL/min) renal function impairment, adjustment of the recommended dose is not required, but patients should be monitored closely for adverse effects of COLCRYS. However, in patients with severe impairment, while the dose does not need to be adjusted for the treatment of gout flares, a treatment course should be repeated no more than once every 2 weeks. For patients with gout flares requiring repeated courses consideration should be given to alternate therapy. For patients undergoing dialysis, the total recommended dose for the treatment of gout flares should be reduced to a single dose of 0.6 mg (1 tablet). For these patients, the treatment course should not be repeated more than once every 2 weeks [see Dose Modification in Renal Impairment (2.5)].
FMF
Although, pharmacokinetics of colchicine in patients with mild (Clcr 50 – 80 mL/min) and moderate (Clcr 30 – 50 mL/min) renal impairment is not known, these patients should be monitored closely for adverse effects of colchicine. Dose reduction may be necessary. In patients with severe renal failure (Clcr less than 30 mL/minute) and end-stage renal disease requiring dialysis, COLCRYS may be started at the dose of 0.3 mg/day. Any increase in dose should be done with adequate monitoring of the patient for adverse effects of COLCRYS [see Pharmacokinetics (12.3) and Dose Modification in Renal Impairment (2.5)].
8.7 Hepatic Impairment
The clearance of colchicine may be significantly reduced and plasma half-life prolonged in patients with chronic hepatic impairment, compared to healthy subjects [see Pharmacokinetics (12.3)].
Prophylaxis of Gout Flares:
For prophylaxis of gout flares in patients with mild to moderate hepatic function impairment, adjustment of the recommended dose is not required, but patients should be monitored closely for adverse effects of colchicine. Dose reduction should be considered for the prophylaxis of gout flares in patients with severe hepatic impairment [see Dose Modification in Hepatic Impairment (2.6)].
Treatment of Gout Flares:
For treatment of gout flares in patients with mild to moderate hepatic function impairment, adjustment of the recommended COLCRYS dose is not required, but patients should be monitored closely for adverse effects of COLCRYS. However, for the treatment of gout flares in patients with severe impairment while the dose does not need to be adjusted, the treatment course should be repeated no more than once every 2 weeks. For these patients, requiring repeated courses for the treatment of gout flares, consideration should be given to alternate therapy [see Dose Modification in Hepatic Impairment (2.6)].
FMF
In patients with severe hepatic disease, dose reduction should be considered with careful monitoring [see Pharmacokinetics (12.3) and Dose Modification in Hepatic Impairment (2.6)].
- 9 DRUG ABUSE AND DEPENDENCE
-
10 OVERDOSAGE
The exact dose of colchicine that produces significant toxicity is unknown. Fatalities have occurred after ingestion of a dose as low as 7 mg over a 4-day period, while other patients have survived after ingesting more than 60 mg. A review of 150 patients who overdosed on colchicine found that those who ingested less than 0.5 mg/kg survived and tended to have milder toxicities, such as gastrointestinal symptoms, whereas those who took 0.5 to 0.8 mg/kg had more severe reactions, such as myelosuppression. There was 100% mortality in those who ingested more than 0.8 mg/kg.
The first stage of acute colchicine toxicity typically begins within 24 hours of ingestion and includes gastrointestinal symptoms, such as abdominal pain, nausea, vomiting, diarrhea, and significant fluid loss, leading to volume depletion. Peripheral leukocytosis may also be seen. Life-threatening complications occur during the second stage, which occurs 24 to 72 hours after drug administration, attributed to multi-organ failure and its consequences. Death is usually a result of respiratory depression and cardiovascular collapse. If the patient survives, recovery of multi-organ injury may be accompanied by rebound leukocytosis and alopecia starting about 1 week after the initial ingestion.
Treatment of colchicine poisoning should begin with gastric lavage and measures to prevent shock. Otherwise, treatment is symptomatic and supportive. No specific antidote is known. Colchicine is not effectively removed by dialysis [see Pharmacokinetics (12.3)].
-
11 DESCRIPTION
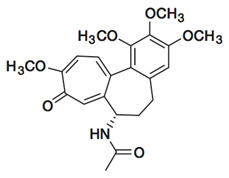
Colchicine is an alkaloid chemically described as (S)N- (5,6,7,9-tetrahydro- 1,2,3, 10-tetramethoxy-9-oxobenzo [alpha] heptalen-7-yl) acetamide with a molecular formula of C22H25NO6 and a molecular weight of 399.4. The structural formula of colchicine is given below.
Colchicine occurs as a pale yellow powder that is soluble in water.
COLCRYS® (colchicine, USP) tablets are supplied for oral administration as purple, film-coated, capsule-shaped tablets (0.1575" × 0.3030"), debossed with 'AR 374' on one side and scored on the other, containing 0.6 mg of the active ingredient colchicine USP. Inactive ingredients: carnauba wax, FD&C blue #2, FD&C red #40, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, pregelatinized starch, sodium starch glycolate, titanium dioxide, and triacetin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism by which COLCRYS exerts its beneficial effect in patients with FMF has not been fully elucidated; however, evidence suggests that colchicine may interfere with the intracellular assembly of the inflammasome complex present in neutrophils and monocytes that mediates activation of interleukin-1β. Additionally, colchicine disrupts cytoskeletal functions through inhibition of β-tubulin polymerization into microtubules, and consequently prevents the activation, degranulation, and migration of neutrophils thought to mediate some gout symptoms.
12.3 Pharmacokinetics
Absorption
In healthy adults, COLCRYS is absorbed when given orally, reaching a mean Cmax of 2.5 ng/mL (range 1.1 to 4.4 ng/mL) in 1 to 2 hours (range 0.5 to 3 hours) after a single dose administered under fasting conditions.
Following oral administration of COLCRYS given as 1.8 mg colchicine over 1 hour to healthy, young adults under fasting conditions, colchicine appears to be readily absorbed, reaching mean maximum plasma concentrations of 6.2 ng/mL at a median 1.81 hours (range: 1.0 to 2.5 hours). Following administration of the non-recommended high-dose regimen (4.8 mg over 6 hours), mean maximal plasma concentrations were 6.8 ng/mL, at a median 4.47 hours (range: 3.1 to 7.5 hours).
After 10 days on a regimen of 0.6 mg twice daily, peak concentrations are 3.1 to 3.6 ng/mL (range 1.6 to 6.0 ng/mL), occurring 1.3 to 1.4 hours post-dose (range 0.5 to 3.0 hours). Mean pharmacokinetic parameter values in healthy adults are shown in Table 5 below.
Table 5 Mean (%CV) Pharmacokinetic Parameters in Healthy Adults Given COLCRYS - * Tmax mean (range)
Cmax (colchicine ng/mL)
Tmax* (h)
Vd/F (L)
CL/F (L/hr)
t1/2 (h)
COLCRYS 0.6 mg Single Dose (N=13)
2.5 (28.7)
1.5 (1.0 – 3.0)
341.5 (54.4)
54.1 (31.0)
--
COLCRYS 0.6 mg b.i.d. × 10 days (N=13)
3.6 (23.7)
1.3 (0.5 – 3.0)
1150 (18.7)
30.3 (19.0)
26.6 (16.3)
CL= Dose/AUC0-t (Calculated from mean values)
Vd = CL/Ke (Calculated from mean values)In some subjects, secondary colchicine peaks are seen, occurring between 3 and 36 hours post-dose and ranging from 39% to 155% of the height of the initial peak. These observations are attributed to intestinal secretion and reabsorption and/or biliary recirculation.
Absolute bioavailability is reported to be approximately 45%.
Administration of COLCRYS with food has no effect on the rate of colchicine absorption, but did decrease the extent of colchicine by approximately 15%. This is without clinical significance.
Distribution
The mean apparent volume of distribution in healthy young volunteers was approximately 5 to 8 L/kg.
Colchicine binding to serum protein is low, 39 ± 5%, primarily to albumin regardless of concentration.
Colchicine crosses the placenta (plasma levels in the fetus are reported to be approximately 15% of the maternal concentration). Colchicine also distributes into breast milk at concentrations similar to those found in the maternal serum [see Pregnancy (8.1) and Nursing Mothers (8.3)].
Metabolism
Colchicine is demethylated to two primary metabolites, 2-O-demethylcolchicine and 3-O-demethylcolchicine (2- and 3-DMC, respectively), and one minor metabolite, 10-O-demethylcolchicine (also known as colchiceine). In vitro studies using human liver microsomes have shown that CYP3A4 is involved in the metabolism of colchicine to 2- and 3-DMC. Plasma levels of these metabolites are minimal (less than 5% of parent drug).
Elimination/Excretion
In healthy volunteers (n=12) 40 – 65% of 1 mg orally administered colchicine was recovered unchanged in urine. Enterohepatic recirculation and biliary excretion are also postulated to play a role in colchicine elimination. Following multiple oral doses (0.6 mg twice daily), the mean elimination half-lives in young healthy volunteers (mean age 25 to 28 years of age) is 26.6 to 31.2 hours. Colchicine is a substrate of P-gp.
Special Populations
There is no difference between men and women in the pharmacokinetic disposition of colchicine.
Elderly: Pharmacokinetics of colchicine has not been determined in elderly patients. A published report described the pharmacokinetics of 1 mg oral colchicine tablet in four elderly women compared to six young healthy males. The mean age of the four elderly women was 83 years (range 75 – 93), mean weight was 47 kg (38 – 61 kg) and mean creatinine clearance was 46 mL/min (range 25 – 75 mL/min). Mean peak plasma levels and AUC of colchicine were two times higher in elderly subjects compared to young healthy males. However, it is possible that the higher exposure in the elderly subjects was due to decreased renal function.
Renal impairment: Pharmacokinetics of colchicine in patients with mild and moderate renal impairment is not known. A published report described the disposition of colchicine (1 mg) in young adult men and women with FMF who had normal renal function or end-stage renal disease requiring dialysis. Patients with end-stage renal disease had 75% lower colchicine clearance (0.17 vs 0.73 L/hr/kg) and prolonged plasma elimination half-life (18.8 hrs vs 4.4 hrs) as compared to subjects with FMF and normal renal function [see Dose Modification in Renal Impairment (2.5) and Renal Impairment (8.6)].
Hepatic impairment: Published reports on the pharmacokinetics of IV colchicine in patients with severe chronic liver disease, as well as those with alcoholic or primary biliary cirrhosis, and normal renal function suggest wide inter-patient variability. In some subjects with mild to moderate cirrhosis, the clearance of colchicine is significantly reduced and plasma half-life prolonged compared to healthy subjects. In subjects with primary biliary cirrhosis, no consistent trends were noted [see Dose Modification in Hepatic Impairment (2.6) and Hepatic Impairment (8.7)]. No pharmacokinetic data are available for patients with severe hepatic impairment (Child-Pugh C).
Drug interactions:
In vitro drug interactions:
In vitro studies in human liver microsomes have shown that colchicine is not an inhibitor or inducer of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4 activity.
In vivo drug interactions:
The effects of co-administration of other drugs with COLCRYS on Cmax, AUC, and Cmin are summarized in Table 6 (effect of other drugs on colchicine) and Table 7 (effect of colchicine on other drugs). For information regarding clinical recommendations, see Table 1 in Dose Modification for Co-administration of Interacting Drugs [see Dose Modification for Co-administration of Interacting Drugs (2.4)].
Table 6 Drug Interactions: Pharmacokinetic Parameters for COLCRYS (colchicine, USP) tablets in the Presence of the Co-Administered Drug Co-administered Drug
Dose of Co-administered Drug (mg)
Dose of COLCRYS (mg)
N
% Change in Colchicine Concentrations from Baseline
(Range: Min - Max)Cmax
AUC0-t
Cyclosporine
100 mg
single-dose0.6 mg
single-dose23
270.0
(62.0 to 606.9)259.0
(75.8 to 511.9)Clarithromycin
250 mg BID,
7 days0.6 mg
single-dose23
227.2
(65.7 to 591.1)281.5
(88.7 to 851.6)Ketoconazole
200 mg BID,
5 days0.6 mg
single-dose24
101.7
(19.6 to 219.0)212.2
(76.7 to 419.6)Ritonavir
100 mg BID,
5 days0.6 mg
single-dose18
184.4
(79.2 to 447.4)296.0
(53.8 to 924.4)Verapamil
240 mg daily,
5 days0.6 mg
single-dose24
40.1
(-47.1 to 149.5)103.3
(-9.8 to 217.2)Diltiazem
240 mg daily,
7 days0.6 mg
single-dose20
44.2
(-46.0 to 318.3)93.4
(-30.2 to 338.6)Azithromycin
500 mg × 1 day, then
250 mg × 4 days0.6 mg
single-dose21
21.6
(-41.7 to 222.0)57.1
(-24.3 to 241.1)Grapefruit Juice
240 mL BID,
4 days0.6 mg
single-dose21
-2.55
(-53.4 to 55.0)-2.36
(-46.4 to 62.2)Estrogen-containing oral contraceptives: In healthy female volunteers given ethinyl estradiol and norethindrone (Ortho-Novum® 1/35) co-administered with COLCRYS (0.6 mg b.i.d. × 14 days), hormone concentrations are not affected.
In healthy volunteers given theophylline co-administered with COLCRYS (0.6 mg b.i.d. × 14 days), theophylline concentrations were not affected.
Table 7 Drug Interactions: Pharmacokinetic Parameters for Co-Administration of Drug in the Presence of COLCRYS (colchicine, USP) tablets - * Conducted in healthy adult females
- † AUCτ
Co-administered Drug
Dose of Co-administered Drug (mg)
Dose of COLCRYS (mg)
N
% Change in Co-Administered Drug Concentrations from Baseline
(Range: Min - Max)Cmax
AUC0-t
Theophylline
300 mg (elixir) single-dose
0.6 mg BID × 14 days
27
1.6
(-30.4 to 23.1)1.6
(-28.5 to 27.1)Ethinyl Estradiol (Ortho-Novum® 1/35)
21-Day Cycle (Active Treatment) + 7-Day Placebo
0.6 mg BID × 14 days
27*
-6.7
(-40.3 to 44.7)-3.0†
(-25.3 to 24.9)Norethindrone (Ortho-Novum® 1/35)
0.94
(-37.3 to 59.4)-1.6†
(-32.0 to 33.7) -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies of colchicine have not been conducted. Due to the potential for colchicine to produce aneuploid cells (cells with an unequal number of chromosomes), there is theoretically an increased risk of malignancy.
Mutagenesis
Colchicine was negative for mutagenicity in the bacterial reverse mutation assay. In a chromosomal aberration assay in cultured human white blood cells, colchicine treatment resulted in the formation of micronuclei. Since published studies demonstrated that colchicine induces aneuploidy from the process of mitotic nondisjunction without structural DNA changes, colchicine is not considered clastogenic, although micronuclei are formed.
Impairment of Fertility
No studies of colchicine effects on fertility were conducted with COLCRYS. However, published nonclinical studies demonstrated that colchicine-induced disruption of microtubule formation affects meiosis and mitosis. Reproductive studies also reported abnormal sperm morphology and reduced sperm counts in males, and interference with sperm penetration, second meiotic division, and normal cleavage in females when exposed to colchicine. Colchicine administered to pregnant animals resulted in fetal death and teratogenicity. These effects were dose dependent, with the timing of exposure critical for the effects on embryofetal development. The nonclinical doses evaluated were generally higher than an equivalent human therapeutic dose, but safety margins for reproductive and developmental toxicity could not be determined.
Case reports and epidemiology studies in human male subjects on colchicine therapy indicated that infertility from colchicine is rare. A case report indicated that azoospermia was reversed when therapy was stopped. Case reports and epidemiology studies in female subjects on colchicine therapy have not established a clear relationship between colchicine use and female infertility. However, since the progression of FMF without treatment may result in infertility, the use of colchicine needs to be weighed against the potential risks.
-
14 CLINICAL STUDIES
The evidence for the efficacy of colchicine in patients with chronic gout is derived from the published literature. Two randomized clinical trials assessed the efficacy of colchicine 0.6 mg twice a day for the prophylaxis of gout flares in patients with gout initiating treatment with urate lowering therapy. In both trials, treatment with colchicine decreased the frequency of gout flares.
The efficacy of a low dosage regimen of oral colchicine (COLCRYS total dose 1.8 mg over 1 hour) for treatment of gout flares was assessed in a multicenter, randomized, double-blind, placebo-controlled, parallel group, 1 week, dose comparison study. Patients meeting American College of Rheumatology criteria for gout were randomly assigned to three groups: high-dose colchicine (1.2 mg, then 0.6 mg hourly × 6 hours [4.8 mg total]); low-dose colchicine (1.2 mg, then 0.6 mg in 1 hour [1.8 mg total] followed by 5 placebo doses hourly); or placebo (2 capsules, then 1 capsule hourly × 6 hours). Patients took the first dose within 12 hours of the onset of the flare and recorded pain intensity (11-point Likert scale) and adverse events over 72 hours. The efficacy of colchicine was measured based on response to treatment in the target joint, using patient self assessment of pain at 24 hours following the time of first dose as recorded in the diary. A responder was one who achieved at least a 50% reduction in pain score at the 24-hour post-dose assessment relative to the pre-treatment score and did not use rescue medication prior to the actual time of 24-hour post-dose assessment.
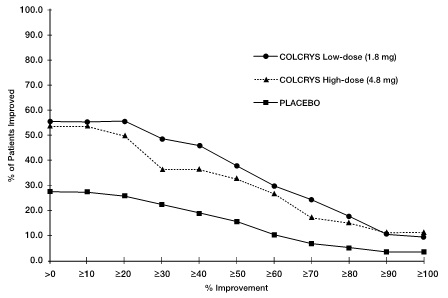
Rates of response were similar for the recommended low-dose treatment group (38%) and the non-recommended high-dose group (33%) but were higher as compared to the placebo group (16%) as shown in Table 8.
Table 8 Number (%) of Responders Based on Target Joint Pain Score at 24 Hours Post First Dose COLCRYS Dose Responders n (%)
Placebo
n (%)
(n=58)% Differences in Proportion
Low-dose
(n=74)High-dose
(n=52)Low-dose vs Placebo
(95% CI)High-dose vs Placebo
(95% CI)28 (38%)
17 (33%)
9 (16%)
22 (8, 37)
17 (1, 33)
Figure 1 below shows the percentage of patients achieving varying degrees of improvement in pain from baseline at 24 hours.
Figure 1
Pain Relief on Low and High Doses of COLCRYS and Placebo (Cumulative)
The evidence for the efficacy of colchicine in patients with FMF is derived from the published literature. Three randomized, placebo-controlled studies were identified. The three placebo-controlled studies randomized a total of 48 adult patients diagnosed with FMF and reported similar efficacy endpoints as well as inclusion and exclusion criteria.
One of the studies randomized 15 patients with FMF to a 6-month crossover study during which 5 patients discontinued due to study non-compliance. The 10 patients completing the study experienced 5 attacks over the course of 90 days while treated with colchicine compared to 59 attacks over the course of 90 days while treated with placebo. Similarly, the second study randomized 22 patients with FMF to a 4-month crossover study during which 9 patients discontinued due to lack of efficacy while receiving placebo or study non-compliance. The 13 patients completing the study experienced 18 attacks over the course of 60 days while treated with colchicine compared to 68 attacks over the course of 60 days while treated with placebo. The third study was discontinued after an interim analysis of 6 of the 11 patients enrolled had completed the study; results could not be confirmed.
Open-label experience with colchicine in adults and children with FMF is consistent with the randomized, controlled trial experience, and was utilized to support information on the safety profile of colchicine and for dosing recommendations.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Repackaged by Aphena Pharma Solutions - TN.
See Repackaging Information for available configurations.
16.1 How Supplied
COLCRYS® (colchicine, USP) tablets 0.6 mg, are purple, film-coated, capsule-shaped tablets, debossed with 'AR 374' on one side and scored on the other side.
Bottles of 30
NDC: 64764-119-07
Bottles of 60
NDC: 64764-119-06
Bottles of 100
NDC: 64764-119-01
Bottles of 250
NDC: 64764-119-03
Bottles of 500
NDC: 64764-119-05
Bottles of 1000
NDC: 64764-119-10
-
17 PATIENT COUNSELING INFORMATION
[See Medication Guide]
17.1 Dosing Instructions
Patients should be advised to take COLCRYS as prescribed, even if they are feeling better. Patients should not alter the dose or discontinue treatment without consulting with their doctor. If a dose of COLCRYS is missed:
- For treatment of a gout flare when the patient is not being dosed for prophylaxis, take the missed dose as soon as possible.
- For treatment of a gout flare during prophylaxis, take the missed dose immediately, wait twelve hours, then resume the previous dosing schedule.
- For prophylaxis without treatment for a gout flare, or FMF, take the dose as soon as possible and then return to the normal dosing schedule. However, if a dose is skipped the patient should not double the next dose.
17.2 Fatal Overdose
Instruct patient that fatal overdoses, both accidental and intentional, have been reported in adults and children who have ingested colchicine. COLCRYS should be kept out of the reach of children.
17.3 Blood Dyscrasias
Patients should be informed that bone marrow depression with agranulocytosis, aplastic anemia, and thrombocytopenia may occur with COLCRYS.
17.4 Drug and Food Interactions
Patients should be advised that many drugs or other substances may interact with COLCRYS and some interactions could be fatal. Therefore, patients should report to their healthcare provider all of the current medications they are taking, and check with their healthcare provider before starting any new medications, particularly antibiotics. Patients should also be advised to report the use of non-prescription medication or herbal products. Grapefruit and grapefruit juice may also interact and should not be consumed during COLCRYS treatment.
17.5 Neuromuscular Toxicity
Patients should be informed that muscle pain or weakness, tingling or numbness in fingers or toes may occur with COLCRYS alone or when it is used with certain other drugs. Patients developing any of these signs or symptoms must discontinue COLCRYS and seek medical evaluation immediately.
-
MEDICATION GUIDE
MEDICATION GUIDE
COLCRYS
(KOL-kris)(colchicine) tablets
Read the Medication Guide that comes with COLCRYS before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or treatment. You and your healthcare provider should talk about COLCRYS when you start taking it and at regular checkups.
What is the most important information I should know about COLCRYS?
COLCRYS can cause serious side effects or death if levels of COLCRYS are too high in your body.
- Taking certain medicines with COLCRYS can cause your level of COLCRYS to be too high, especially if you have kidney or liver problems.
- Tell your healthcare provider about all your medical conditions, including if you have kidney or liver problems. Your dose of COLCRYS may need to be changed.
- Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements.
- Even medicines that you take for a short period of time, such as antibiotics, can interact with COLCRYS and cause serious side effects or death.
- Talk to your healthcare provider or pharmacist before taking any new medicine.
- Especially tell your healthcare provider if you take:
- atazanavir sulfate (Reyataz®)
- cyclosporine (Neoral®, Gengraf®, Sandimmune®)
- fosamprenavir (Lexiva®) with ritonavir
- indinavir (Crixivan®)
- ketoconazole (Nizoral®)
- nefazodone (Serzone®)
- ritonavir (Norvir®)
- telithromycin (Ketek®)
- clarithromycin (Biaxin®)
- darunavir (Prezista®)
- fosamprenavir (Lexiva®)
- itraconazole (Sporanox®)
- lopinavir/ritonavir (Kaletra®)
- nelfinavir mesylate (Viracept®)
- saquinavir mesylate (Invirase®)
- tipranavir (Aptivus®)
Ask your healthcare provider or pharmacist if you are not sure if you take any of the medicines listed above. This is not a complete list of all the medicines that can interact with COLCRYS.
- Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
- Keep COLCRYS out of the reach of children.
What is COLCRYS?
COLCRYS is a prescription medicine used to:
- prevent and treat gout flares in adults
- treat familial Mediterranean fever (FMF) in adults and children age four or older
COLCRYS is not a pain medicine and it should not be taken to treat pain related to other conditions unless specifically prescribed for those conditions.
Who should not take COLCRYS?
Do not take COLCRYS if you have liver or kidney problems and you take certain other medicines. Serious side effects, including death, have been reported in these patients even when taken as directed. See "What is the most important information I should know about COLCRYS?"
What should I tell my healthcare provider before starting COLCRYS?
See "What is the most important information I should know about COLCRYS?"
Before you take COLCRYS tell your healthcare provider about all your medical conditions including if you:
- have liver or kidney problems
- are pregnant or plan to become pregnant. It is not known if COLCRYS will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breast-feeding or plan to breast-feed. COLCRYS passes into your breast milk. You and your healthcare provider should decide if you will take COLCRYS or breast-feed. If you take COLCRYS and breast-feed, you should talk to your child's healthcare provider about how to watch for side effects in your child.
Tell your healthcare provider about all the medicines you take, including ones that you may only be taking for a short time, such as antibiotics. See "What is the most important information I should know about COLCRYS?" Do not start a new medicine without talking to your healthcare provider.
Using COLCRYS with certain other medicines, such as cholesterol-lowering medications and digoxin, can affect each other causing serious side effects. Your healthcare provider may need to change your dose of COLCRYS. Talk to your healthcare provider about whether the medications you are taking might interact with COLCRYS, and what side effects to look for.
How should I take COLCRYS?
- Take COLCRYS exactly as your healthcare provider tells you to take it. If you are not sure about your dosing, call your healthcare provider.
- COLCRYS can be taken with or without food.
- If you take too much COLCRYS go to the nearest hospital emergency room right away.
- Do not stop taking COLCRYS even if you start to feel better, unless your healthcare provider tells you.
- Your healthcare provider may do blood tests while you take COLCRYS.
- If you take COLCRYS daily and you miss a dose, then take it as soon as you remember. If it is almost time for your next dose, just skip the missed dose. Take the next dose at your regular time. Do not take 2 doses at the same time.
- If you have a gout flare while taking COLCRYS daily, report this to your healthcare provider.
What should I avoid while taking COLCRYS?
- Avoid eating grapefruit or drinking grapefruit juice while taking COLCRYS. It can increase your chances of getting serious side effects.
What are the possible side effects of COLCRYS?
COLCRYS can cause serious side effects or even cause death. See "What is the most important information I should know about COLCRYS?"
Get medical help right away, if you have:
- Muscle weakness or pain
- Numbness or tingling in your fingers or toes
- Unusual bleeding or bruising
- Increased infections
- Feel weak or tired
- Pale or gray color to your lips, tongue, or palms of your hands
- Severe diarrhea or vomiting
Gout Flares: The most common side effect of COLCRYS in people who have gout flares is diarrhea.
FMF: The most common side effects of COLCRYS in people who have FMF are abdominal pain, diarrhea, nausea and vomiting.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of COLCRYS. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store COLCRYS?
- Store COLCRYS at room temperature between 68° and 77°F (20° to 25°C).
- Keep COLCRYS in a tightly closed container.
- Keep COLCRYS out of the light.
Keep COLCRYS and all medicines out of the reach of children.
General Information about COLCRYS
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use COLCRYS for a condition for which it was not prescribed. Do not give COLCRYS to other people, even if they have the same symptoms that you have. It may harm them. This Medication Guide summarizes the most important information about COLCRYS. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about COLCRYS that is written for healthcare professionals.
For more information, go to www.COLCRYS.com or call 1-877-825-3327.
What are the ingredients in COLCRYS?
Active Ingredient: Colchicine
Inactive Ingredients: carnauba wax, FD&C blue #2, FD&C red #40, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, pregelatinized starch, sodium starch glycolate, titanium dioxide, and triacetin.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
All trademarks are the property of their respective owners.
Distributed by:
Takeda Pharmaceuticals America, Inc.
Deerfield, IL 60015
Rev 01, June 2012
-
Repackaging Information
Please reference the How Supplied section listed above for a description of individual tablets or capsules. This drug product has been received by Aphena Pharma - TN in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
Count 0.6mg 9000 43353-854-09 Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20140423SC - PRINCIPAL DISPLAY PANEL - 0.6mg
-
INGREDIENTS AND APPEARANCE
COLCRYS
colchicine tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 43353-854(NDC:64764-119) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Colchicine (UNII: SML2Y3J35T) (Colchicine - UNII:SML2Y3J35T) Colchicine 0.6 mg Inactive Ingredients Ingredient Name Strength carnauba wax (UNII: R12CBM0EIZ) FD&C blue no. 2 (UNII: L06K8R7DQK) FD&C red no. 40 (UNII: WZB9127XOA) hypromellose, unspecified (UNII: 3NXW29V3WO) lactose monohydrate (UNII: EWQ57Q8I5X) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) polydextrose (UNII: VH2XOU12IE) polyethylene glycol, unspecified (UNII: 3WJQ0SDW1A) starch, corn (UNII: O8232NY3SJ) sodium starch glycolate type A potato (UNII: 5856J3G2A2) titanium dioxide (UNII: 15FIX9V2JP) triacetin (UNII: XHX3C3X673) Product Characteristics Color PURPLE (Film Coated) Score 2 pieces Shape OVAL (capsule-shaped) Size 8mm Flavor Imprint Code AR;374 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43353-854-09 9000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022352 09/01/2009 Labeler - Aphena Pharma Solutions - Tennessee, LLC (128385585) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions - Tennessee, LLC 128385585 Repack(43353-854)
Trademark Results [Colcrys]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COLCRYS 77978156 3716922 Live/Registered |
TAKEDA PHARMACEUTICALS U.S.A., INC. 2008-12-18 |
 COLCRYS 77636223 3842336 Live/Registered |
Takeda Pharmaceuticals U.S.A., Inc. 2008-12-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.