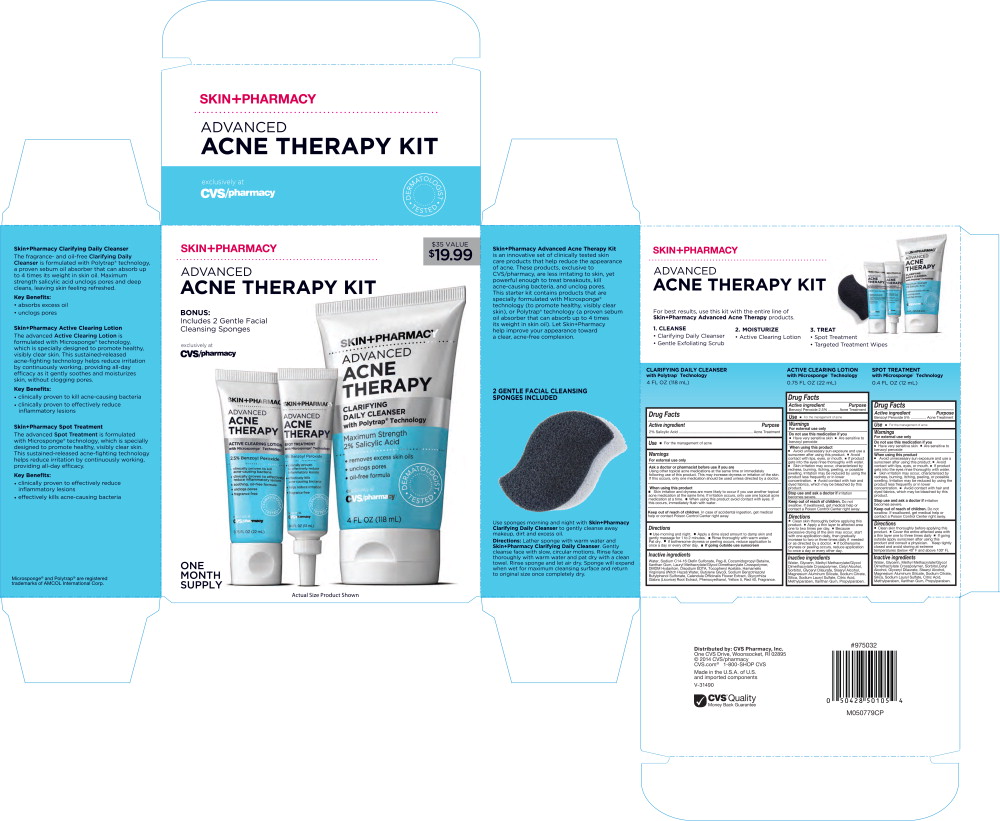
SKINPHARMACY ADVANCED ACNE THERAPY KIT- salicylic acid, benzoyl peroxide kit
SkinPharmacy Advanced Acne Therapy Kit by
Drug Labeling and Warnings
SkinPharmacy Advanced Acne Therapy Kit by is a Otc medication manufactured, distributed, or labeled by CVS Health, AMCOL Health & Beauty Solutions, Inc. DBA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
-
Warnings
For external use only
Ask a doctor or pharmacist before use if you are
Using other topical acne medications at the same time or immediately following use of this product. This may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
When using this product
- Skin irritation and dryness are more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- When using this product avoid contact with eyes. If this occurs, immediately flush with water.
- Directions
-
Inactive ingredients
Water, Sodium C14-16 Olefin Sulfonate, Peg-8, Cocamidopropyl Betaine, Xanthan Gum, Lauryl Methacrylate/Glycol Dimethacrylate Crosspolymer, DMDM Hydantoin, Disodium EDTA, Tocopheryl Acetate, Hamamelis Virginiana (Witch Hazel) Water, Butylene Glycol, Sodium Benzotriazolyl Butylphenol Sulfonate, Calendula Officinalis Flower Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Phenoxyethanol, Yellow 5, Red 40, Fragrance.
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- Avoid unnecessary sun exposure and use a sunscreen after using this product.
- Avoid contact with lips, eyes, or mouth.
- If product gets into eyes rinse thoroughly with water.
- Skin irritation may occur, characterized by redness, burning, itching, peeling, or possible swelling. Irritation may be reduced by using the product less frequently or in lower concentration.
- Avoid contact with hair and dyed fabrics, which may be bleached by this product.
-
Directions
- Clean skin thoroughly before applying this product.
- Apply a thin layer to affected area one to two times per day.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two to three time daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- Avoid unnecessary sun exposure and use a sunscreen after using this product.
- Avoid contact with lips, eyes, or mouth.
- If product gets into eyes rinse thoroughly with water.
- Skin irritation may occur, characterized by redness, burning, itching, peeling, or possible swelling. Irritation may be reduced by using the product less frequently or in lower concentration.
- Avoid contact with hair and dyed fabrics, which may be bleached by this product.
-
Directions
- Clean skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily.
- If going outside apply sunscreen after using this product and consult a physician. Keep tightly closed and avoid storing at extreme temperatures (below 40°F and above 100°F).
-
Inactive ingredients
Water, Glycerin, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, Sorbitol, Cetyl Alcohol, Glyceryl Dilaurate, Stearyl Alcohol, Magnesium Aluminum Silicate, Sodium Citrate, Silica, Sodium Lauryl Sulfate, Citric Acid, Methylparaben, Xanthan Gum, Propylparaben.
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2014 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
Made in the U.S.A. of U.S. and imported components
V-31490
CVS® Quality
Money Back Guarantee
#975032
M050779CP
- Principal Display Panel - Carton Label
- Principal Display Panel - Clarifying Daily Cleanser Tube Label
-
Principal Display Panel - Active Clearing Lotion Tube Label
SKIN+PHARMACY
ADVANCED
ACNE
THERAPYACTIVE CLEARING LOTION
with Microsponge® Technology- clinically proven to kill acne-causing bacteria
- clinically proven to effectively reduce inflammatory lesions
- soothing, oil-free formula
- unclogs pores
- fragrance free
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacy0.75 FL OZ (22 mL)
-
Principal Display Panel - Spot Treatment Tube Label
SKIN+PHARMACY
ADVANCED
ACNE
THERAPYSPOT TREATMENT
with Microsponge® Technology5% Benzoyl Peroxide
- clinically proven to effectively reduce inflammatory lesions
- effectively kills acne-causing bacteria
- helps reduce irritation
- fragrance free
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacy0.4 FL OZ (12 mL)
-
INGREDIENTS AND APPEARANCE
SKINPHARMACY ADVANCED ACNE THERAPY KIT
salicylic acid, benzoyl peroxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69842-082 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69842-082-01 1 in 1 CARTON; Type 0: Not a Combination Product 10/01/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 118 mL Part 2 1 TUBE 22 mL Part 3 1 TUBE 12 mL Part 1 of 3 SKINPHARMACY ADVANCED ACNE THERAPY CLARIFYING DAILY CLEANSER
salicylic acid liquidProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength salicylic acid (UNII: O414PZ4LPZ) (salicylic acid - UNII:O414PZ4LPZ) salicylic acid 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) sodium C14-16 olefin sulfonate (UNII: O9W3D3YF5U) polyethylene glycol 4000 (UNII: 4R4HFI6D95) cocamidopropyl betaine (UNII: 5OCF3O11KX) Xanthan gum (UNII: TTV12P4NEE) DMDM Hydantoin (UNII: BYR0546TOW) edetate disodium (UNII: 7FLD91C86K) .alpha.-tocopherol acetate, DL- (UNII: WR1WPI7EW8) Hamamelis virginiana flower water (UNII: 222MYC9QUV) Butylene glycol (UNII: 3XUS85K0RA) Calendula Officinalis flower (UNII: P0M7O4Y7YD) licorice (UNII: 61ZBX54883) Phenoxyethanol (UNII: HIE492ZZ3T) FD&C Yellow No. 5 (UNII: I753WB2F1M) FD&C red no. 40 (UNII: WZB9127XOA) Lauryl Methacrylate/Glycol Dimethacrylate Crosspolymer (UNII: EX0F4CZ66H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 118 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 10/01/2014 Part 2 of 3 SKINPHARMACY ADVANCED ACNE THERAPY ACTIVE CLEARING
benzoyl peroxide lotionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength benzoyl peroxide (UNII: W9WZN9A0GM) (benzoyl peroxide - UNII:W9WZN9A0GM) benzoyl peroxide 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) glycerin (UNII: PDC6A3C0OX) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) cetyl alcohol (UNII: 936JST6JCN) sorbitol (UNII: 506T60A25R) glyceryl dilaurate (UNII: MFL3ZIE8SK) stearyl alcohol (UNII: 2KR89I4H1Y) magnesium aluminum silicate (UNII: 6M3P64V0NC) sodium citrate (UNII: 1Q73Q2JULR) silicon dioxide (UNII: ETJ7Z6XBU4) sodium lauryl sulfate (UNII: 368GB5141J) citric acid monohydrate (UNII: 2968PHW8QP) methylparaben (UNII: A2I8C7HI9T) xanthan gum (UNII: TTV12P4NEE) propylparaben (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 22 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 10/01/2014 Part 3 of 3 SKINPHARMACY ADVANCED ACNE THERAPY SPOT TREATMENT
benzoyl peroxide lotionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength benzoyl peroxide (UNII: W9WZN9A0GM) (benzoyl peroxide - UNII:W9WZN9A0GM) benzoyl peroxide 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) glycerin (UNII: PDC6A3C0OX) methyl methacrylate/glycol dimethacrylate crosspolymer (UNII: EG97988M5Q) cetyl alcohol (UNII: 936JST6JCN) sorbitol (UNII: 506T60A25R) glyceryl dilaurate (UNII: MFL3ZIE8SK) stearyl alcohol (UNII: 2KR89I4H1Y) magnesium aluminum silicate (UNII: 6M3P64V0NC) sodium citrate (UNII: 1Q73Q2JULR) silicon dioxide (UNII: ETJ7Z6XBU4) sodium lauryl sulfate (UNII: 368GB5141J) citric acid monohydrate (UNII: 2968PHW8QP) methylparaben (UNII: A2I8C7HI9T) xanthan gum (UNII: TTV12P4NEE) propylparaben (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 12 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 10/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 10/01/2014 Labeler - CVS Health (062312574) Registrant - AMCOL Health & Beauty Solutions, Inc. DBA (872684803) Establishment Name Address ID/FEI Business Operations AMCOL Health & Beauty Solutions, Inc. DBA 872684803 ANALYSIS(69842-082) , MANUFACTURE(69842-082) , LABEL(69842-082) , PACK(69842-082)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.