RYCLORA- dexchlorpheniramine maleate liquid
RYCLORA by
Drug Labeling and Warnings
RYCLORA by is a Prescription medication manufactured, distributed, or labeled by CARWIN PHARMACEUTICAL ASSOCIATES, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each 5 mL (teaspoonful) contains: Dexchlorpheniramine Maleate, USP 2 mg Dexchlorpheniramine Maleate, USP, an antihistamine agent, is a white, odorless crystalline powder that is freely soluble in water. The molecular formula is C16H19ClN2 ∙ C4H4O4, designated chemically as (+)-2-[p-Chloro-α-[2-(dimethylamino)ethyl]benzyl] pyridine maleate (1:1).
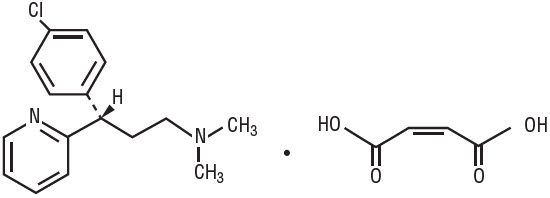
M.W. = 390.86
Inactive Ingredients: Citric acid, cherry flavoring, FD&C Red No. 40, glycerin, menthol, methylparaben, propylene glycol, propylparaben, purified water, sodium citrate dihydrate, and sugar.
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
Perennial and seasonal allergic rhinitis
Vasomotor rhinitis
Allergic conjunctivitis due to inhalant allergens and foods
Mild, uncomplicated allergic skin manifestations of urticaria and angioedema
Amelioration of allergic reactions to blood or plasma
Dermographism
As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
-
CONTRAINDICATIONS
Use in Nursing Mothers
Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
Use in Lower Respiratory Disease
Antihistamines should NOT be used to treat lower respiratory tract symptoms including asthma.
Antihistamines are also contraindicated in the following conditions:
- Hypersensitivity to dexchlorpheniramine maleate or other antihistamines of similar chemical structure
- Monoamine oxidase inhibitor therapy (See Drug Interaction section)
-
WARNINGS
Antihistamines should be used with considerable caution in patients with:
- Narrow angle glaucoma
- Stenosing peptic ulcer
- Pyloroduodenal obstruction
- Symptomatic prostatic hypertrophy
- Bladder neck obstruction
Use in Children
In infants and children, especially, antihistamines in overdosage may cause hallucinations, convulsions, or death.
As in adults, antihistamines may diminish mental alertness in children. In the young child, particularly, they may produce excitation.
Use in Pregnancy
Experience with this drug in pregnant women is inadequate to determine whether there exists a potential for harm to the developing fetus.
Use with CNS Depressants
RYCLORA™ Oral Solution has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc.).
- PRECAUTIONS
-
ADVERSE REACTIONS
- General: Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose and the throat.
- Cardiovascular System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
- Hematologic System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
- Nervous System: Sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesias, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, hysteria, neuritis, convulsions.
- G.I. System: Epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation.
- G.U. System: Urinary frequency, difficult urination, urinary retention, early menses.
- Respiratory System: Thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness.
Call your doctor for medical advice about side effects. You may voluntarily report side effects to FDA at 1-800-FDA-1088. Questions or comments? Call Carwin Pharmaceutical Associates, LLC at 1-844-700-5011.
-
OVERDOSAGE
Antihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in children. Atropine-like signs and symptoms—dry mouth, fixed, dilated pupils, flushing, and gastrointestinal symptoms may also occur.
If vomiting has not occurred spontaneously the patient should be induced to vomit. This is best done by having the patient drink a glass of water or milk after which the patient should be made to gag. Precautions against aspiration must be taken, especially in infants and children.
Saline cathartics, such as milk of magnesia, draw water into the bowel by osmosis and therefore, are valuable for their action in rapid dilution of bowel content.
Stimulants should not be used.
Vasopressors may be used to treat hypotension.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
RYCLORA™ Oral Solution is supplied as a red colored, cherry flavored liquid in the following sizes:
4 fl oz (118 mL), NDC: 15370-150-04
16 fl oz (473 mL), NDC: 15370-150-16
0.7 fl oz (20mL), NDC: 15370-150-99 (as physician sample) - SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
NDC: 15370-150-16
RyClora™
(dexchlorpheniramine maleate)Oral Solution, USP
2 mg/5 mLCherry Flavor
Rx Only
16 fl. oz (473 mL)carwin
PHARMACEUTICAL ASSOCIATES
-
INGREDIENTS AND APPEARANCE
RYCLORA
dexchlorpheniramine maleate liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 15370-150 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dexchlorpheniramine Maleate (UNII: B10YD955QW) (Dexchlorpheniramine - UNII:3Q9Q0B929N) Dexchlorpheniramine Maleate 2 mg in 5 mL Inactive Ingredients Ingredient Name Strength Citric Acid Monohydrate (UNII: 2968PHW8QP) FD&C Red No. 40 (UNII: WZB9127XOA) glycerin (UNII: PDC6A3C0OX) menthol, unspecified form (UNII: L7T10EIP3A) methylparaben (UNII: A2I8C7HI9T) propylene glycol (UNII: 6DC9Q167V3) propylparaben (UNII: Z8IX2SC1OH) water (UNII: 059QF0KO0R) trisodium citrate dihydrate (UNII: B22547B95K) sucrose (UNII: C151H8M554) Product Characteristics Color RED Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 15370-150-16 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/07/2018 2 NDC: 15370-150-04 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/07/2018 3 NDC: 15370-150-99 20 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/07/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202520 10/07/2018 Labeler - CARWIN PHARMACEUTICAL ASSOCIATES, LLC (079217215)
Trademark Results [RYCLORA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RYCLORA 87782094 not registered Live/Pending |
Carwin Pharmaceutical Associates, LLC 2018-02-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.