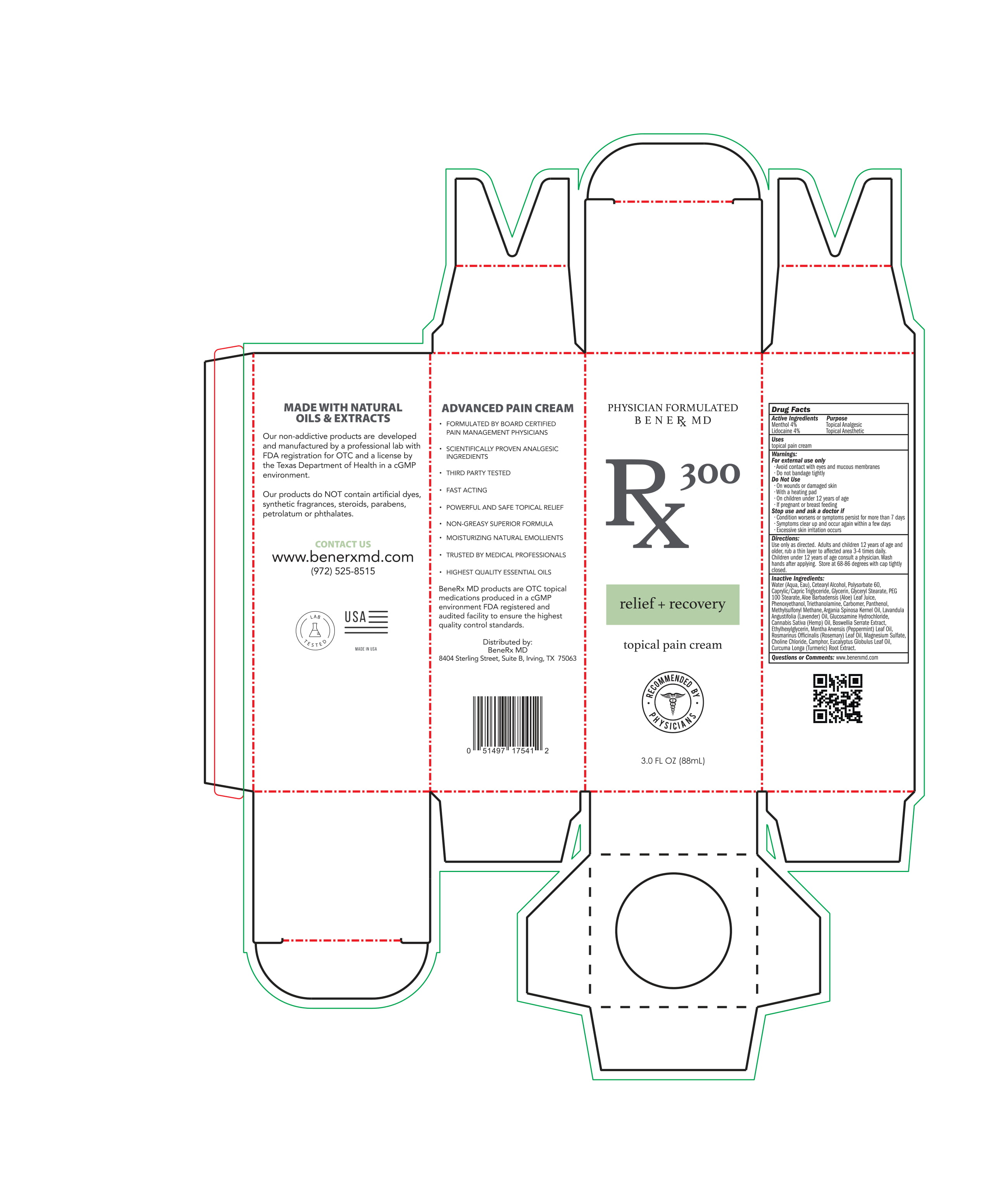
BeneRX MD RX300 Topical Pain Cream
RX300 Topical Pain Cream by
Drug Labeling and Warnings
RX300 Topical Pain Cream by is a Otc medication manufactured, distributed, or labeled by BENERX LLC, Dhaliwal Pharmaceuticals Laboratories LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
RX300 TOPICAL PAIN CREAM PAIN CREAM- menthol, lidocaine creamÂ
BENERX LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BeneRX MD RX300 Topical Pain Cream
Do Not Use
- On wounds or damaged skin
- With a heating pad
- On children under 12 year of age
- If pregnant or breast feeding
Warnings:
For external use only
- Avoid contact with eyes and mucous memberanes
- Do not bandage tightly
Stop use and ask a doctor if
- Condition worsens or symptoms persist for more than 7 days
- Symptoms clear up and occur again within a few days
- Excessive skin irritation occurs
Stop use and ask a doctor if:
- Condition worsens or symptoms persists for more than 7 days
- Excessive skin irritation occurs
- Symptoms clear up and occur again within a few days
Directions
- Use as directed
- Adults and children 12 years of age and older, rub a thin layer to affected area 3-4 times daily
- Children under 12 years of age consult a physician
- wash hands after applying
Water (Aqua, Eau)
cetearyl alcohol
polysorbate 60
Caprylic/ Capric Triglyceride
Glycerin
Glyceryl Stearate
Peg 100 Stearate
Aloe Barbadensis (Aloe) Leaf Juice
Phenoxyethanol
Triethanolamine
Carbomer
Panthenol
Methylsulfonyl Methane
Argania Spinosa Kernel Oil
Lavandula Angustifolia (Lavender) Oil
Glucosamine hydrocholride
Cannabis Sativa Hemp Oil
Boswellia Serrate Extract
Ethylhexylglycerin
Mentha Anensis (Peppermint) Leaf Oil
Rosmarinus Officinalis (Rosemary) Leaf Oil
Magnesium Sulfate
Choline Chloride
Camphor
Eucalyptus Globulous Leaf Oil
Curcuma Longa (Turmeric) Root Extract
| RX300 TOPICAL PAIN CREAMÂ
PAIN CREAM
menthol, lidocaine cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler -Â BENERX LLC (113572537) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dhaliwal Pharmaceuticals Laboratories LLC | 116933772 | manufacture(79884-001) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.