Brightening TRIO by ULTRACEUTICALS PTY LIMITED / NANOPHASE TECHNOLOGIES CORPORATION Brightening TRIO
Brightening TRIO by
Drug Labeling and Warnings
Brightening TRIO by is a Otc medication manufactured, distributed, or labeled by ULTRACEUTICALS PTY LIMITED, NANOPHASE TECHNOLOGIES CORPORATION. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
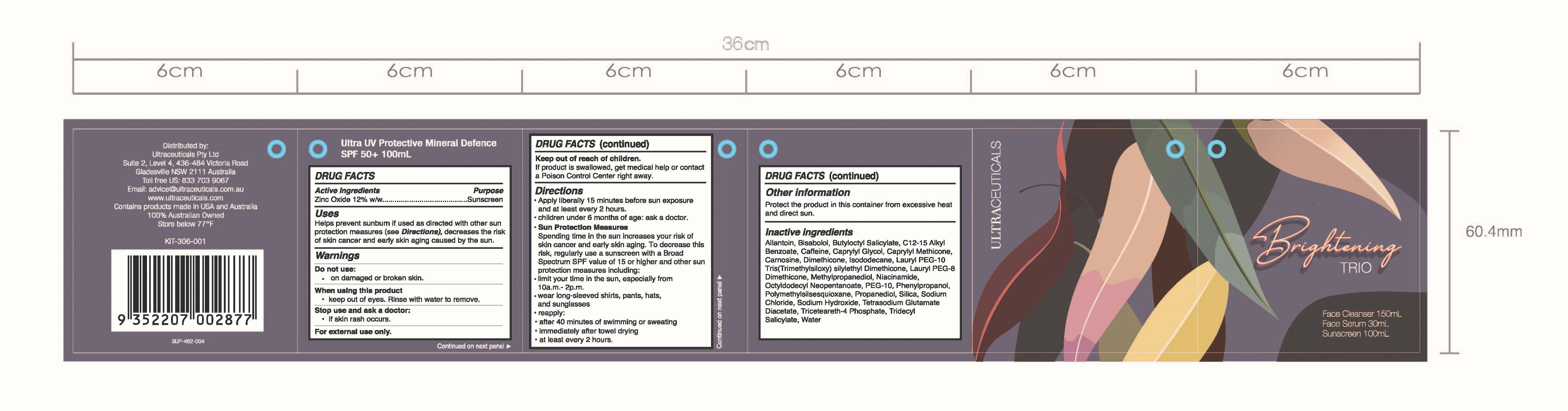
BRIGHTENING TRIO- zinc oxide
ULTRACEUTICALS PTY LIMITED
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Brightening TRIO
Uses
Helps prevent sunburn if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
Do not use:
on damaged or broken skin.
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor:
if skin rash occurs.
For external use only.
Directions
- Apply liberally 15 minutes before sun exposure and at least every 2 hours.
- children under 6 months of age: ask a doctor.
- Sun Protection Measures Spending time in the sun increases your risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit your time in the sun, especially from 10a.m.-2p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- reapply:
- after 40 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours.
Allantoin, Bisabolol, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Caffeine, Caprylyl Glycol, Caprylyl Methicone, Carnosine, Dimethicone, Isododecane, Lauryl PEG-10 Tris(Trimethylsiloxy) silylethyl Dimethicone, Lauryl PEG-8 Dimethicone. Methylpropanediol, Niacinamide, Octyldodecyl Neopentanoate, PEG-10, Phenylpropanol, Polymethylsilsesquioxane, Propanediol, Silica, Sodium Chloride, Sodium Hydroxide, Tetrasodium Glutamate Diacetate, Triceteareth-4 Phosphate, Tridecyl Salicylate, Water.
Brightening TRIO
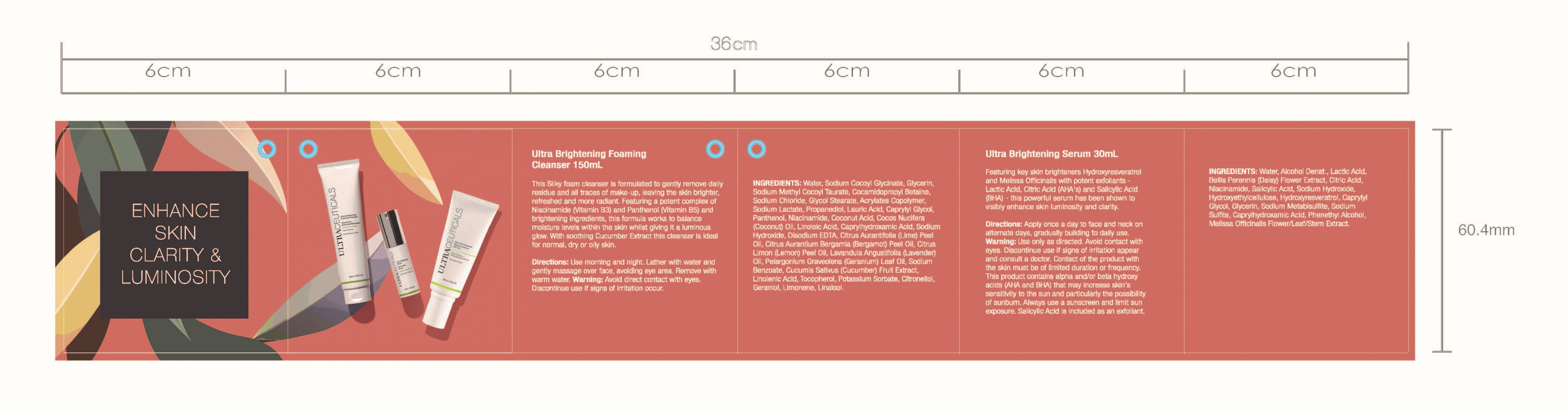
Face Cleanser 150mL
Face Serum 30 mL
Sunscreen 100mL
Ultra UV Protective Mineral Defence SPF50+ 100mL
Distributed by:
Ultraceuticals Pty Ltd
Suite 2, Level 4, 436-484 Victoria Road
Gladesville NSW 2111 Australia
Toll free US: 833 703 9067
Email: advice@ultraceuticlas.com.au
www.ultraceuticals.com
Contains products made in USA and Australia
100% Australian Owned
Store below 77F
KIT-306-001
| BRIGHTENING TRIO
zinc oxide kit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - ULTRACEUTICALS PTY LIMITED (744068995) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NANOPHASE TECHNOLOGIES CORPORATION | 623502044 | manufacture(10028-028) | |