Sanatos Lozenges by Pharmadel LLC SanaTos EX Lozenges
Sanatos Lozenges by
Drug Labeling and Warnings
Sanatos Lozenges by is a Otc medication manufactured, distributed, or labeled by Pharmadel LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
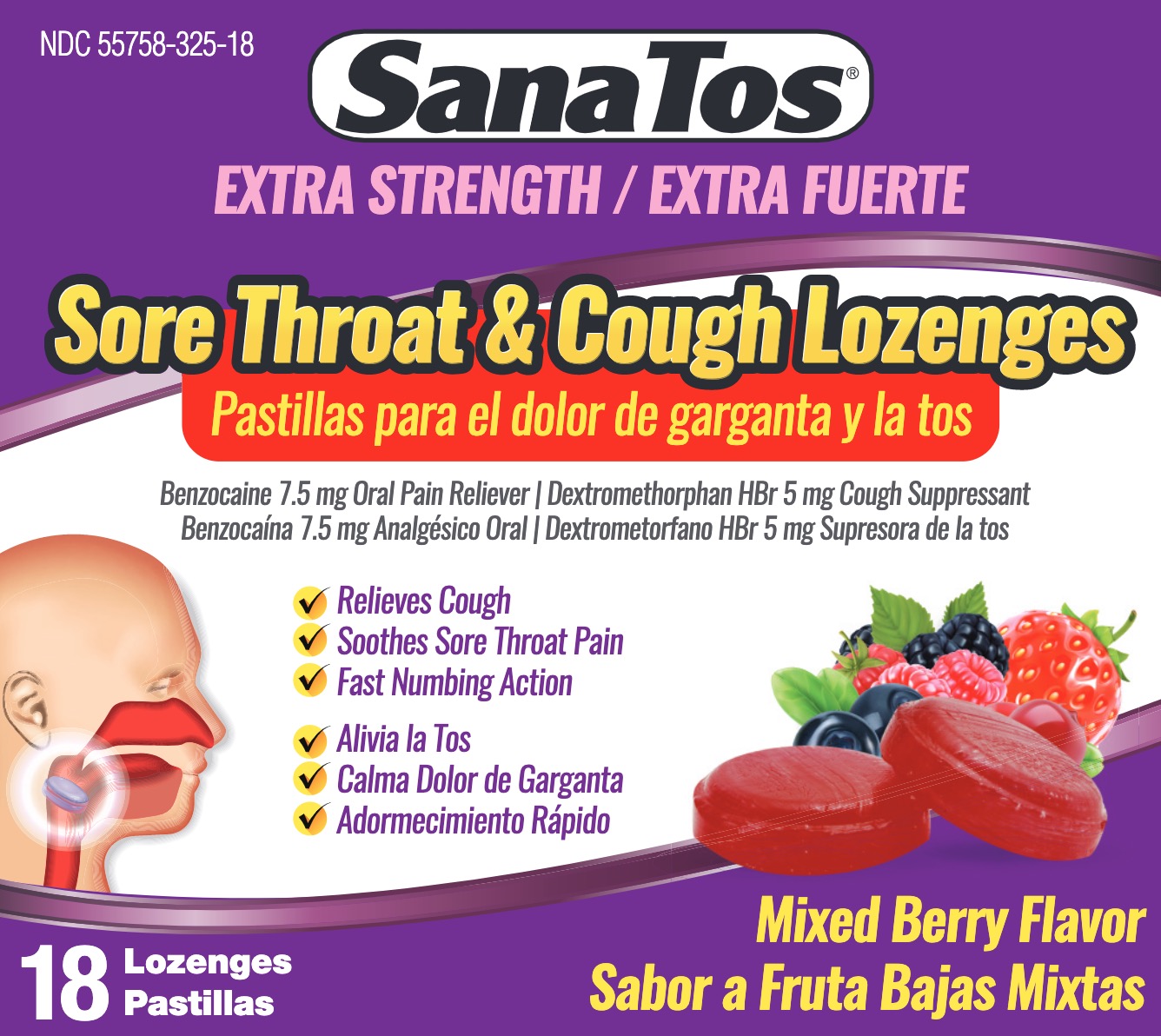
SANATOS LOZENGES EX- benzocaine, dextromethorphan hbr lozenge
Pharmadel LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SanaTos EX Lozenges
Uses
For the temporary relief of occasional
- minor irritation
- sore throat
- sore mouth
- cough as may occur with a cold
- pain associated with canker sores
Warnings
Methemoglobinemia warning: use of this product may cause methemoglobinemia, a serous condition that must be treated promptly because it reduces the amount of oygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert:
do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics.
Sore throat warning:
if sore throat is severe persists for more than 2 days, is accompanied or followed by high fever, headache, rash, nausea or vomiting, consult a doctor promptly. These may be serious.
Do not use
- if you are taking a perscription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains as MAOI, asl a doctor or pharmacist before taking this product.
- do not use for teething
- in children under 2 years of age
Ask a doctor before use if you have
- a persistent or chronic cough such as occurs with smoking, asthma or emphysema
- a cough that is accompanied by excessive phlegm (mucus0
Directions
- dissolve the lozenges slowly in the mouth. May repeat every 4 hours as needed or as direected by doctor.
| Age | Dose |
| adult and childrens 12 years and over | take 2 lozenges |
| children 2 to 12 years age | take 1 lozenge |
| children under 2 years of age | do not use |
| SANATOS LOZENGES
EX
benzocaine, dextromethorphan hbr lozenge |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Pharmadel LLC (030129680) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.