TUKOL MULTI SYMPTOM COLD- dextromethorphan hydrobromide, guaifenesin, phenylephrine hydrochloride liquid
Tukol Multi Symptom Cold by
Drug Labeling and Warnings
Tukol Multi Symptom Cold by is a Otc medication manufactured, distributed, or labeled by Genomma Lab USA, Inc., AptaPharma Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
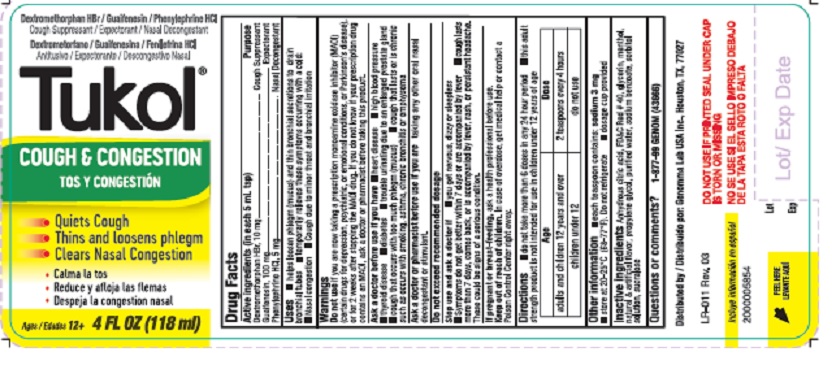
- Active ingredients
- Purposes
- Keep out of reach of children
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product. -
Ask a doctor before use
Ask a doctor or pharmacist before use
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- cough that occurs with too much phlegm mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
Ask a doctor or pharmacist before use if you are
taking any other oral nasal decongestant or stimulant.
When using this product do not use more than directed. - Stop use and ask a doctor if
- If pregnant or breast-feeding
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
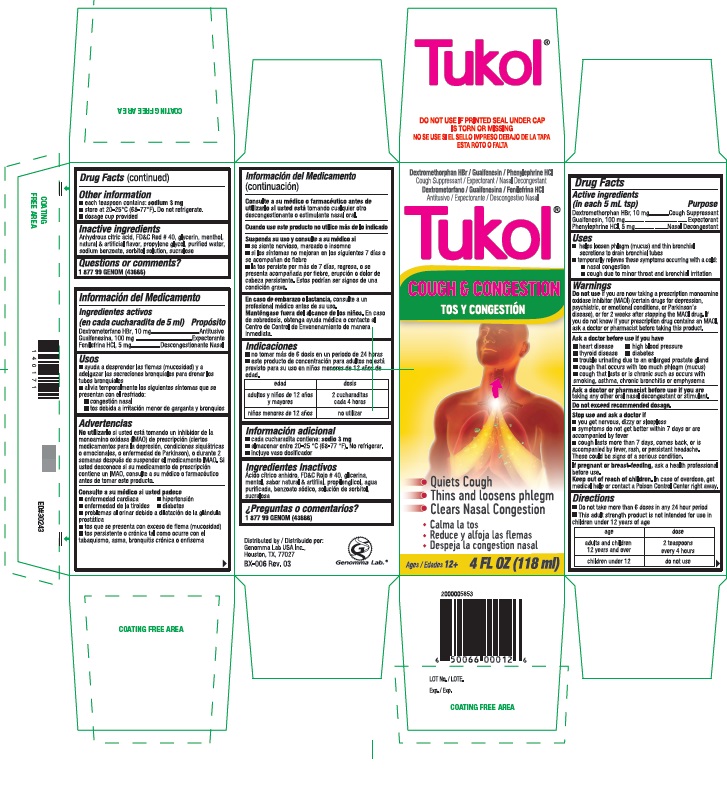
Product Label Tukol 504 DPL
Tukol®
DO NOT USE IF PRINTED SEAL UNDER CAP
IS TORN OR MISSING
Dextromethorphan HBr / Guaifenesin / Phenylephrine HCL
Expectorant/Cough Suppressant/Nasal Decongestant
Tukol®
Cough & Congestion
- Quiets Cough
- Thins and loosens phlegm
- Clears Nasal Congestion
Ages/ 12+ 4 FL OZ (118 mL)
2000005853
6 50066 00012 6
LOT No.
Exp.Distributed by
Genomma Lab USA Inc.Houston, TX 77027
BX-006 Rev. 03
Genoma Lab.®
rege
-
INGREDIENTS AND APPEARANCE
TUKOL MULTI SYMPTOM COLD
dextromethorphan hydrobromide, guaifenesin, phenylephrine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50066-504 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL (UNII: L7T10EIP3A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50066-504-24 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/15/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/15/2012 Labeler - Genomma Lab USA, Inc. (832323534) Registrant - AptaPharma Inc. (790523323) Establishment Name Address ID/FEI Business Operations AptaPharma Inc. 790523323 manufacture(50066-504)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.