CITROMA- magesium citrate liquid
citroma by
Drug Labeling and Warnings
citroma by is a Otc medication manufactured, distributed, or labeled by Duane Reade, Vi-Jon. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
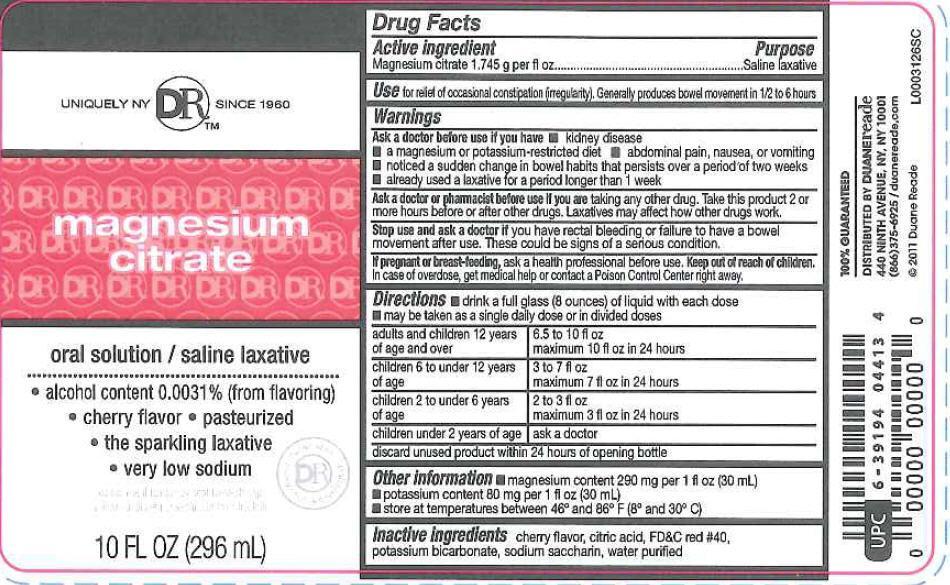
- Active Ingredient
- Purpose
- use
- Warnings
- Ask A Doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- Stop use and
- if pregnant or breast-feeding
- Keep out of reach of children.
-
Directions
- drink a full glass (8 ounces) of liquid with each dose
- may be taken as a single daily dose or in divided doses
adults and children 12 years of age and over - 6.5 to 10 fl oz in 24 hours
children 6 to under 12 years of age - 3 to 7 fl oz maximum 7 fl oz in 24 hours
children 2 to under 6 years of age - 2 to 3 fl oz maximum of 3 fl oz in 24 hours
children under 2 years of age - ask a doctor
discard unused product within 24 hours of opening bottle
- other information
- Inactive ingredients
- Adverse Reaction
- principal display panel
-
INGREDIENTS AND APPEARANCE
CITROMA
magesium citrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 67732-693 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM CITRATE (UNII: RHO26O1T9V) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CITRATE 1.745 g in 29.6 mL Inactive Ingredients Ingredient Name Strength CHERRY (UNII: BUC5I9595W) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM BICARBONATE (UNII: HM5Z15LEBN) WATER (UNII: 059QF0KO0R) FD&C RED NO. 40 (UNII: WZB9127XOA) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67732-693-38 296 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 08/20/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 08/20/1995 Labeler - Duane Reade (011988995) Registrant - Vi-Jon (790752542) Establishment Name Address ID/FEI Business Operations Vi-Jon 790752542 manufacture(67732-693)
Trademark Results [citroma]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CITROMA 79069355 not registered Dead/Abandoned |
Jungbunzlauer Ladenburg GmbH 2009-02-06 |
 CITROMA 74202768 1704520 Live/Registered |
VI-JON, INC. 1991-09-12 |
 CITROMA 71266472 0247578 Dead/Expired |
1928-05-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.