Jack Black The Supreme Shave by Jack Black L.L.C / Swiss American CDMO
Jack Black The Supreme Shave by
Drug Labeling and Warnings
Jack Black The Supreme Shave by is a Otc medication manufactured, distributed, or labeled by Jack Black L.L.C, Swiss American CDMO. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
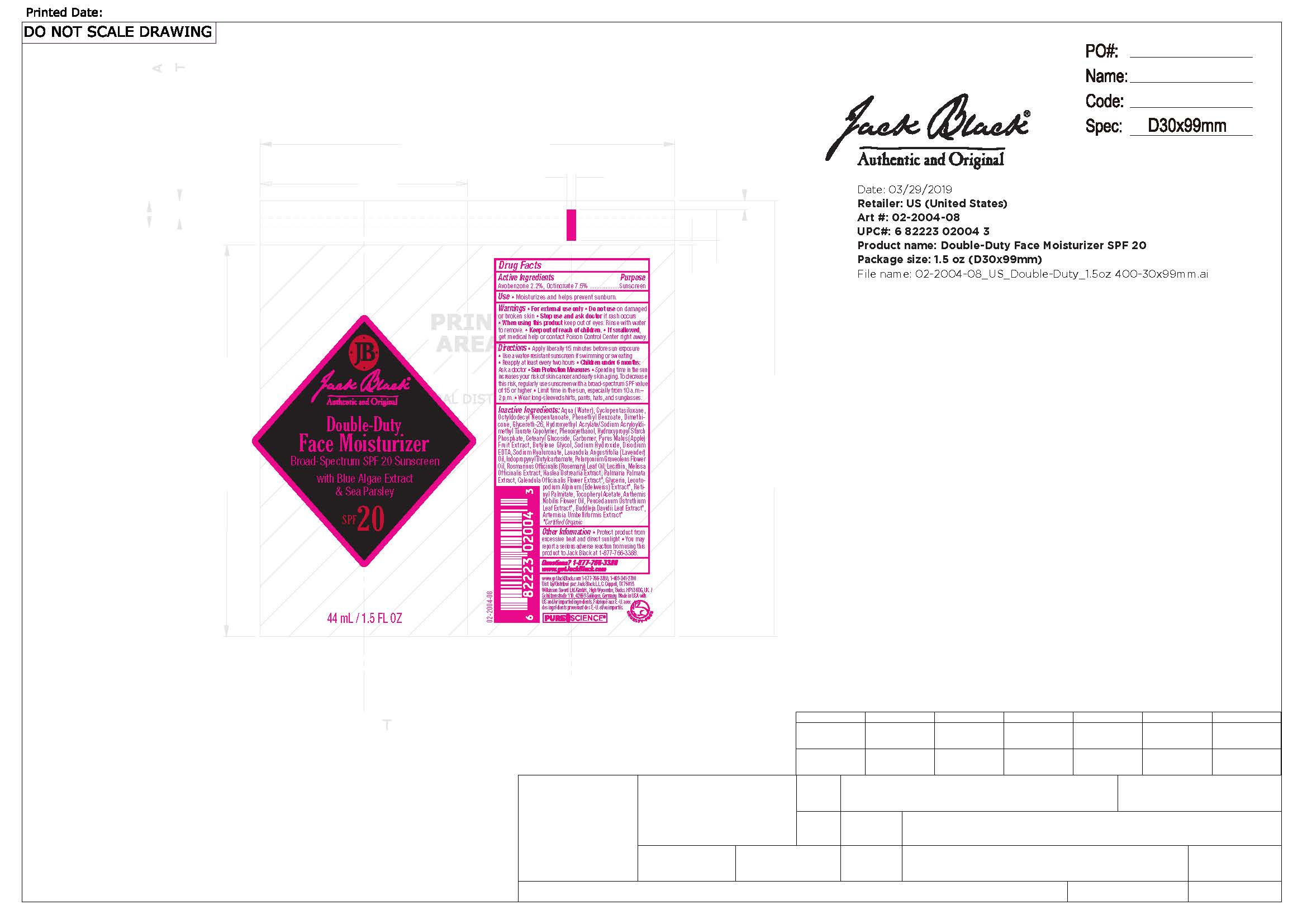
JACK BLACK THE SUPREME SHAVE- avobenzone, octinoxate lotion
Jack Black L.L.C
----------
Keep out of reach of children.
If swallowed, get medical help or contact Poison Control Center right away.
Directions
Apply liberally after cleansing and shaving and 15 minutes before sun exposure. Use a water-resistant sunscreen if swimming or sweating. Reapply at least every two hours. Children under 6 months: Ask a doctor. Sun Protection Measures Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, use sunscreen with a broad spectrum SPF 15 or higher. Limit time in the sun, especially from 10 a.m. – 2 p.m. Wear long-sleeved shirts, pants, hats, and sunglasses.
Inactive Ingredients
Water, Cyclopentasiloxane, Octyldodecyl Neopentanoate, Phenethyl Benzoate, Dimethicone, Glycereth-26, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Phenoxyethanol, Hydroxypropyl Starch Phosphate, Cetearyl Glucoside, Carbomer, Pyrus Malus (Apple) Fruit Extract, Butylene Glycol, Sodium Hydroxide, Disodium EDTA, Sodium Hyaluronate, Lavandula Angustifolia (Lavender) Oil, Iodopropynyl Butylcarbamate, Pelargonium Graveolens Flower Oil, Rosmarinus Officinalis (Rosemary) Leaf Oil, Lecithin, Melissa Officinalis Extract, Haslea Ostrearia Extract, Palmaria Palmata Extract, Calendula Officinalis Flower Extract*, Glycerin, Leontopodium Alpinum (Edelweiss) Extract*, Retinyl Palmitate, Tocopheryl Acetate, Anthemis Nobilis Flower Oil, Peucedanum Ostruthium Leaf Extract*, Buddleja Davidii Leaf Extract*, Artemisia Umbelliformis Extract*.
* Certified Organic
| JACK BLACK THE SUPREME SHAVE
avobenzone, octinoxate lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Jack Black L.L.C (847024036) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Swiss American CDMO | 080170933 | manufacture(66738-631) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.