ORPHENADRINE CITRATE- orphenadrine citrate tablet, extended release
Orphenadrine Citrate by
Drug Labeling and Warnings
Orphenadrine Citrate by is a Prescription medication manufactured, distributed, or labeled by H.J. Harkins Company, Inc., Impax Laboratories Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Orphenadrine citrate is the citrate salt of orphenadrine (2-dimethylaminoethyl 2-methyl-benzhydryl ether citrate). It occurs as a white, crystalline powder having a bitter taste. It is practically odorless; sparingly soluble in water, slightly soluble in alcohol. Each orphenadrine citrate tablet contains 100 mg orphenadrine citrate, USP. Orphenadrine citrate tablets also contain ethylcellulose NF, povidone USP, lactose monohydrate NF, and magnesium stearate NF.
- ACTIONS
-
INDICATIONS
Orphenadrine citrate is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute painful musculo-skeletal conditions. The mode of action of the drug has not been clearly identified, but may be related to its analgesic properties. Orphenadrine citrate does not directly relax tense skeletal muscles in man.
-
CONTRAINDICATIONS
Contraindicated in patients with glaucoma, pyloric or duodenal obstruction, stenosing peptic ulcers, prostatic hypertrophy or obstruction of the bladder neck, cardiospasm (megaesophagus) and myasthenia gravis. Contraindicated in patients who have demonstrated a previous hypersensitivity to the drug.
-
WARNINGS
Some patients may experience transient episodes of light-headedness, dizziness or syncope. Orphenadrine citrate may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; ambulatory patients should therefore be cautioned accordingly.
-
PREGNANCY
Pregnancy category C
Safe use of orphenadrine citrate has not been established with respect to adverse effects upon fetal development. Therefore, orphenadrine citrate should be used in women of childbearing potential and particularly during early pregnancy only when in the judgement of the physician the potential benefits outweigh the possible hazards.
- USAGE IN CHILDREN
-
PRECAUTIONS
Confusion, anxiety and tremors have been reported in a few patients receiving propoxyphene and orphenadrine concomitantly. As these symptoms may be simply due to an additive effect, reduction of dosage and/or discontinuation of one or both agents is recommended in such cases.
Orphenadrine citrate should be used with caution in patients with tachycardia, cardiac decompensation, coronary insufficiency, or cardiac arrhythmias.
Safety of continuous long-term therapy with orphenadrine has not been established. Therefore, if orphenadrine is prescribed for prolonged use, periodic monitoring of blood, urine and liver function values is recommended.
-
ADVERSE REACTIONS
Adverse reactions of orphenadrine are mainly due to the mild anticholinergic action of orphenadrine, and are usually associated with higher dosage. Dryness of the mouth is usually the first adverse effect to appear. When the daily dose is increased, possible adverse effects include: tachycardia, palpitation, urinary hesitancy or retention, blurred vision, dilation of pupils, increased ocular tension, weakness, nausea, vomiting, headache, dizziness, constipation, drowsiness, hypersensitivity reactions, pruritus, hallucinations, agitation, tremor, gastric irritation, and rarely urticaria and other dermatoses. Infrequently, an elderly patient may experience some degree of mental confusion. These adverse reactions can usually be eliminated by reduction in dosage. Very rare cases of aplastic anemia associated with the use of orphenadrine tablets have been reported. No causal relationship has been established.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
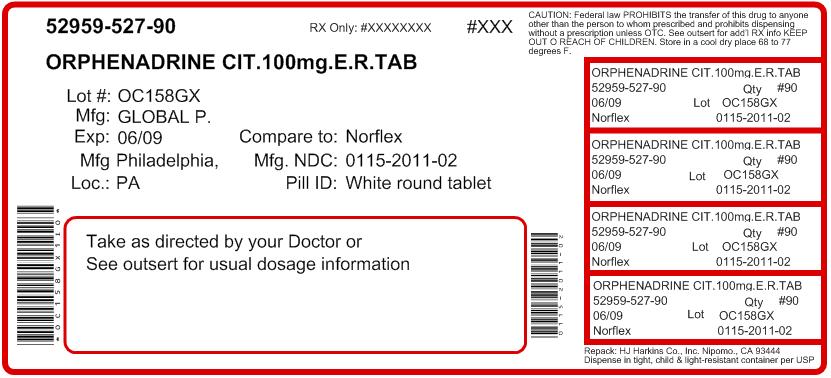
- PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
ORPHENADRINE CITRATE
orphenadrine citrate tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52959-527(NDC:0115-2011) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ORPHENADRINE CITRATE (UNII: X0A40N8I4S) (ORPHENADRINE - UNII:AL805O9OG9) ORPHENADRINE CITRATE 100 mg Inactive Ingredients Ingredient Name Strength POVIDONE (UNII: FZ989GH94E) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score no score Shape ROUND (convex) Size 9mm Flavor Imprint Code G;2011 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52959-527-30 30 in 1 BOTTLE 2 NDC: 52959-527-60 60 in 1 BOTTLE 3 NDC: 52959-527-20 20 in 1 BOTTLE 4 NDC: 52959-527-10 10 in 1 BOTTLE 5 NDC: 52959-527-14 14 in 1 BOTTLE 6 NDC: 52959-527-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040368 06/23/2000 Labeler - H.J. Harkins Company, Inc. (147681894) Establishment Name Address ID/FEI Business Operations Impax Laboratories Inc 790947167 MANUFACTURE Establishment Name Address ID/FEI Business Operations H.J. Harkins Company, Inc. 147681894 REPACK
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.