TUSSIN DM- dextromethorphan hbr, guaifenesin solution
Tussin DM by
Drug Labeling and Warnings
Tussin DM by is a Otc medication manufactured, distributed, or labeled by Chain Drug Marketing Association, Inc., LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each 20 mL)
- Purpose
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
QC
Quality
Choice ®NDC: 83324-324-04
Compare to the
Active Ingredients in
Robitussin® Maximum
Strength Cough+Chest
Congestion DM*Tussin DM
Maximum Strength
Dextromethorphan HBr
Guaifenesin
Cough Suppressant
ExpectorantOral Solution
Controls Cough
Relieves Chest Congestion
Thins & Loosens MucusFor Ages 12 Years & Over
Menthol-Berry Flavored
4 FL OZ (118 mL)
TAMPER EVIDENT: DO NOT USE IF PRINTED
NECK WRAP IS BROKEN OR MISSING*This product is not manufactured or distributed by
Haleon US Holdings LLC, owner of the registered
trademark Robitussin® MAXIMUM STRENGTH
Cough+Chest Congestion DM.50844 ORG032503036
Distributed by CDMA, Inc.
Novi, MI 48375
www.qualitychoice.com
Questions: 800-935-2362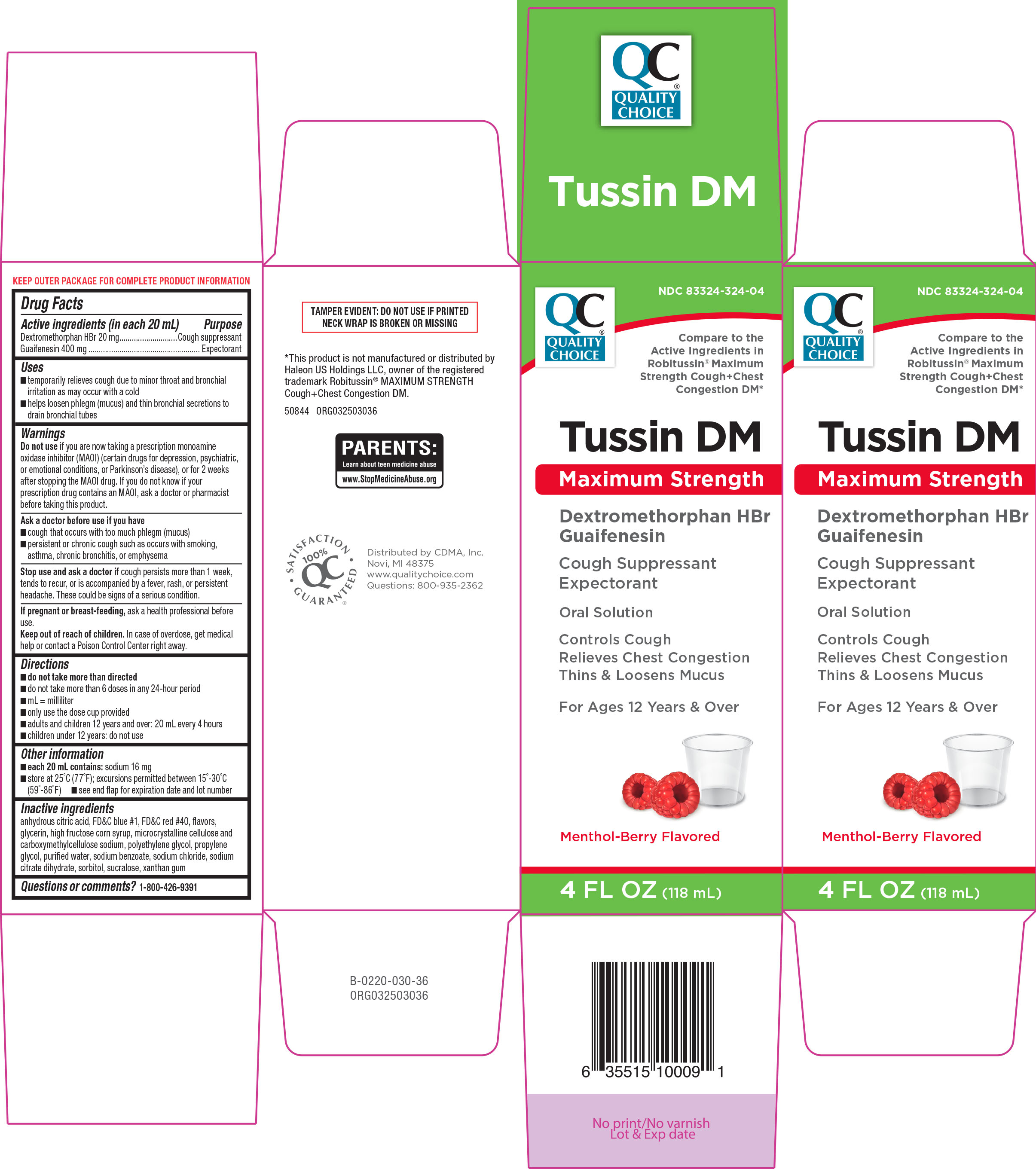
Quality Choice 44-030
-
INGREDIENTS AND APPEARANCE
TUSSIN DM
dextromethorphan hbr, guaifenesin solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 83324-324 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color red Score Shape Size Flavor MENTHOL, BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83324-324-04 1 in 1 CARTON 09/04/2025 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 83324-324-08 1 in 1 CARTON 09/04/2025 2 237 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 09/04/2025 Labeler - Chain Drug Marketing Association, Inc. (011920774) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 manufacture(83324-324) , pack(83324-324)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.