DOTTI- estradiol patch, extended release
DOTTI by
Drug Labeling and Warnings
DOTTI by is a Prescription medication manufactured, distributed, or labeled by Bryant Ranch Prepack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DOTTI safely and effectively. See full prescribing information for DOTTI.
DOTTI® (estradiol transdermal system)
Initial U.S. Approval: 1996WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, PROBABLE DEMENTIA, and BREAST CANCER
See full prescribing information for complete boxed warning.
Estrogen-Alone Therapy
- There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens (5.2)
- The Women’s Health Initiative (WHI) estrogen-alone substudy reported increased risks of stroke and deep vein thrombosis (DVT) (5.1)
- The WHI Memory Study (WHIMS) estrogen-alone ancillary study of WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age and older (5.3)
- Do not use estrogen-alone therapy for the prevention of cardiovascular disease or dementia (5.1, 5.3)
Estrogen Plus Progestin Therapy
- The WHI estrogen plus progestin substudy reported increased risks of DVT, pulmonary embolism (PE), stroke and myocardial infarction (MI) (5.1)
- The WHI estrogen plus progestin substudy reported increased risks of invasive breast cancer (5.2)
- The WHIMS estrogen plus progestin ancillary study of WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age and older (5.3)
- Do not use estrogen plus progestogen therapy for the prevention of cardiovascular disease or dementia (5.1, 5.3)
RECENT MAJOR CHANGES
Warnings and Precautions, Malignant Neoplasms (5.2) 11/2023
INDICATIONS AND USAGE
DOTTI is an estrogen indicated for:
- Treatment of moderate to severe vasomotor symptoms due to menopause (1.1)
- Treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause (1.2)
Limitations of Use
When prescribing solely for the treatment of moderate to severe vaginal atrophy, first consider the use of topical vaginal products.
- Treatment of hypoestrogenism due to hypogonadism, castration, or primary ovarian failure (1.3)
- Prevention of postmenopausal osteoporosis (1.4)
Limitations of Use
When prescribing solely for the prevention of postmenopausal osteoporosis, first consider the use of non-estrogen medications. Consider estrogen therapy only for women at significant risk of osteoporosis.
DOSAGE AND ADMINISTRATION
- Start therapy with DOTTI 0.0375 mg/day applied to the skin twice weekly for the treatment of moderate to severe vasomotor symptoms due to menopause or moderate to severe symptoms of vulvar and vaginal atrophy symptoms due to menopause. Dosage adjustment should be guided by the clinical response (2.1, 2.2, 2.3)
- Start therapy with DOTTI 0.025 mg/day for the prevention of postmenopausal osteoporosis (2.4)
- Place DOTTI on a clean, dry area of the lower abdomen or buttocks. Do not apply DOTTI to the breasts (2.5)
DOSAGE FORMS AND STRENGTHS
Transdermal system, USP: 0.025 mg/day, 0.0375 mg/day, 0.05 mg/day, 0.075 mg/day, and 0.1 mg/day (3)
CONTRAINDICATIONS
- Undiagnosed abnormal genital bleeding (4, 5.2)
- Breast cancer or a history of breast cancer (4, 5.2)
- Estrogen-dependent neoplasia (4, 5.2)
- Active DVT, PE or a history of these conditions (4, 5.1)
- Active arterial thromboembolic disease (for example, stroke and MI), or a history of these conditions (4, 5.1)
- Known anaphylactic reaction, or angioedema, or hypersensitivity with DOTTI (4, 5.15)
- Hepatic impairment or disease (4, 5.10)
- Protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions (≥10%) with DOTTI are: headache, breast tenderness, nasopharyngitis, sinusitis, sinus headache, upper respiratory tract infection, back pain, depression, and irregular vaginal bleeding or spotting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
Inducers and/or inhibitors of CYP3A4 may affect estrogen drug metabolism and decrease or increase the estrogen plasma concentration. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, PROBABLE DEMENTIA, and BREAST CANCER
RECENT MAJOR CHANGES
1 INDICATIONS AND USAGE
1.1 Treatment of Moderate to Severe Vasomotor Symptoms Due to Menopause
1.2 Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy Due to Menopause
1.3 Treatment of Hypoestrogenism Due to Hypogonadism, Castration, or Primary Ovarian Failure
1.4 Prevention of Postmenopausal Osteoporosis
2 DOSAGE AND ADMINISTRATION
2.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause
2.2 Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause
2.3 Hypoestrogenism Due to Hypogonadism, Castration, or Primary Ovarian Failure
2.4 Prevention of Postmenopausal Osteoporosis
2.5 Application Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Disorders
5.2 Malignant Neoplasms
5.3 Probable Dementia
5.4 Gallbladder Disease
5.5 Hypercalcemia
5.6 Visual Abnormalities
5.7 Addition of a Progestogen When a Woman Has Not Had a Hysterectomy
5.8 Elevated Blood Pressure
5.9 Exacerbation of Hypertriglyceridemia
5.10 Hepatic Impairment and/or Past History of Cholestatic Jaundice
5.11 Exacerbation of Hypothyroidism
5.12 Fluid Retention
5.13 Hypocalcemia
5.14 Exacerbation of Endometriosis
5.15 Severe Anaphylactic/Anaphylactoid Reactions and Angioedema
5.16 Exacerbation of Other Conditions
5.17 Laboratory Tests
5.18 Drug-Laboratory Test Interactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Effects on Vasomotor Symptoms in Postmenopausal Women
14.2 Effects on Bone Mineral Density in Postmenopausal Women
14.3 Women’s Health Initiative Studies
14.4 Women’s Health Initiative Memory Study
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, PROBABLE DEMENTIA, and BREAST CANCER
Estrogen-Alone Therapy
Endometrial Cancer
There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens. Adding a progestogen to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer. Perform adequate diagnostic measures, including directed or random endometrial sampling when indicated, to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding [see Warnings and Precautions (5.2)].
Cardiovascular Disorders and Probable Dementia
The Women's Health Initiative (WHI) estrogen-alone substudy reported increased risks of stroke and deep vein thrombosis (DVT) in postmenopausal women (50 to 79 years of age) during 7.1 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg]-alone, relative to placebo [see Warnings and Precautions (5.1), and Clinical Studies (14.3)].
The WHI Memory Study (WHIMS) estrogen-alone ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age and older during 5.2 years of treatment with daily CE (0.625 mg)-alone, relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see Warnings and Precautions (5.3), Use in Specific Populations (8.5), and Clinical Studies (14.4)].
Do not use estrogen-alone therapy for the prevention of cardiovascular disease or dementia [see Warnings and Precautions (5.1, 5.3), and Clinical Studies (14.3, 14.4)].
Only daily oral 0.625 mg CE was studied in the estrogen-alone substudy of WHI. Therefore, the relevance of the WHI findings regarding adverse cardiovascular events and dementia to lower CE doses, other routes of administration, or other estrogen-alone products is not known. Without such data, it is not possible to definitively exclude these risks or determine the extent of these risks for other products. Discuss with your patient the benefits and risks of estrogen-alone therapy, taking into account her individual risk profile.
Prescribe estrogens with or without progestogens at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Estrogen Plus Progestin Therapy
Cardiovascular Disorders and Probable Dementia
The WHI estrogen plus progestin substudy reported increased risks of DVT, pulmonary embolism (PE), stroke and myocardial infarction (MI) in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral CE (0.625 mg) combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo [see Warnings and Precautions (5.1), and Clinical Studies (14.3)].
The WHIMS estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age and older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see Warnings and Precautions (5.3), Use in Specific Populations (8.5), and Clinical Studies (14.4)].
Do not use estrogen plus progestogen therapy for the prevention of cardiovascular disease or dementia [see Warnings and Precautions (5.1, 5.3), and Clinical Studies (14.3, 14.4)].
Breast CancerThe WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer [see Warnings and Precautions (5.2), and Clinical Studies (14.3)].
Only daily oral 0.625 mg CE and 2.5 mg MPA were studied in the estrogen plus progestin substudy of the WHI. Therefore, the relevance of the WHI findings regarding adverse cardiovascular events, dementia and breast cancer to lower CE plus other MPA doses, other routes of administration, or other estrogen plus progestogen products is not known. Without such data, it is not possible to definitively exclude these risks or determine the extent of these risks for other products. Discuss with your patient the benefits and risks of estrogen plus progestogen therapy, taking into account her individual risk profile.
Prescribe estrogens with or without progestogens at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman. - 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Generally, when estrogen is prescribed for a postmenopausal woman with a uterus, consider addition of a progestogen to reduce the risk of endometrial cancer. Generally, a woman without a uterus does not need to use a progestogen in addition to her estrogen therapy. In some cases, however, hysterectomized women who have a history of endometriosis may need a progestogen [see Warnings and Precautions (5.2, 5.14)].
Use estrogen-alone or in combination with a progestogen at the lowest effective dose and the shortest duration consistent with treatment goals and risks for the individual woman. Reevaluate postmenopausal women periodically as clinically appropriate to determine whether treatment is still necessary.
2.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause
Start therapy with DOTTI 0.0375 mg per day applied to the skin twice weekly. Make dosage adjustments based on the clinical response. Initiate DOTTI at once in a woman not currently taking oral estrogens or in a woman switching from another estradiol transdermal therapy. In women who are currently taking oral estrogens, initiate treatment with DOTTI 1 week after withdrawal of oral hormone therapy, or sooner if menopausal symptoms reappear in less than 1 week. Attempts to taper or discontinue DOTTI at 3 to 6 month intervals.
Give DOTTI continuously in a woman who does not have an intact uterus. In a woman with an intact uterus, give DOTTI on a cyclic schedule (for example, 3 weeks on DOTTI followed by 1 week off DOTTI).
2.2 Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause
Start therapy with DOTTI 0.0375 mg per day applied to the skin twice weekly. Dosage adjustment should be guided by the clinical response. Attempts to taper or discontinue DOTTI at 3 to 6 month intervals.
In women not currently taking oral estrogens or in women switching from another estradiol transdermal therapy, treatment with DOTTI may be initiated at once. In women who are currently taking oral estrogens, initiate treatment with DOTTI 1 week after withdrawal of oral hormone therapy, or sooner if menopausal symptoms reappear in less than 1 week.
Give DOTTI continuously in a woman who does not have an intact uterus. In a woman with an intact uterus, give DOTTI on a cyclic schedule (for example, 3 weeks on DOTTI followed by 1 week off DOTTI).
2.4 Prevention of Postmenopausal Osteoporosis
Start therapy with DOTTI 0.025 mg per day applied to the skin twice weekly.
In women not currently taking oral estrogens or in women switching from another estradiol transdermal therapy, treatment with DOTTI may be initiated at once. In women who are currently taking oral estrogens, initiate treatment with DOTTI 1 week after withdrawal of oral hormone therapy, or sooner if menopausal symptoms reappear in less than 1 week.
DOTTI may be given continuously in a woman who does not have an intact uterus. In a woman with an intact uterus, DOTTI may be given on a cyclic schedule (for example, 3 weeks on DOTTI followed by 1 week off DOTTI).
2.5 Application Instructions
Place the adhesive side of DOTTI on a clean, dry area of the trunk of the body (including the abdomen or buttocks). Do not apply DOTTI to the breasts.
Replace DOTTI twice weekly. Rotate the sites of application, with an interval of at least 1 week allowed between applications to a particular site. Select an area that is not oily, damaged, or irritated. Avoid the waistline, since tight clothing may rub the system off. Apply the system immediately after opening the pouch and removing the protective liner. Press the system firmly in place with the palm of the hand for about 10 seconds, making sure there is good contact, especially around the edges.
In the event that a system falls off, reapply the same system or apply a new system to another location. In either case, continue the original treatment schedule. If a woman has forgotten to apply DOTTI, have her apply a new system as soon as possible. Apply the new system on the original treatment schedule. The interruption of treatment in women taking DOTTI might increase the likelihood of breakthrough bleeding, spotting and recurrence of symptoms.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
DOTTI is contraindicated in women with any of the following conditions:
- Undiagnosed abnormal genital bleeding [see Warnings and Precautions (5.2)].
- Breast cancer or a history of breast cancer [see Warnings and Precautions (5.2)].
- Estrogen-dependent neoplasia [see Warnings and Precautions (5.2)].
- Active DVT, PE, or a history of these conditions [see Warnings and Precautions (5.1)].
- Active arterial thromboembolic disease (for example, stroke and MI), or a history of these conditions [see Warnings and Precautions (5.1)].
- Known anaphylactic reaction, or angioedema, or hypersensitivity to DOTTI
- Hepatic impairment or disease
- Protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Disorders
Increased risks of stroke and DVT are reported with estrogen-alone therapy. Increased risks of PE, DVT, stroke, and MI are reported with estrogen plus progestin therapy. Immediately discontinue estrogen with or without progestogen therapy if any of these occur or are suspected.
Manage appropriately any risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus).
Stroke
The WHI estrogen-alone substudy reported a statistically significant increased risk of stroke in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 strokes per 10,000 women-years, respectively). The increase in risk was demonstrated in year 1 and persisted [see Clinical Studies (14.3)]. Immediately discontinue estrogen-alone therapy if a stroke occurs or is suspected.
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
The WHI estrogen plus progestin substudy reported a statistically significant increased risk of stroke in women 50 to 79 years of age receiving CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 stokes per 10,000 women-years) [see Clinical Studies (14.3)]. The increase in risk was demonstrated after the first year and persisted. 1 Immediately discontinue estrogen plus progestogen therapy if a stroke occurs or is suspected.
Coronary Heart Disease
The WHI estrogen-alone substudy reported no overall effect on coronary heart disease (CHD) events (defined as nonfatal MI, silent MI, or CHD death) in women receiving estrogen-alone compared to placebo2 [see Clinical Studies (14.3)].
Subgroup analyses of women 50 to 59 years of age, who were less than 10 years since menopause, suggest a reduction (not statistically significant) in CHD events in those women receiving daily CE (0.625 mg)–alone compared to placebo (8 versus 16 per 10,000 women-years).1
The WHI estrogen plus progestin substudy reported an increased risk (not statistically significant) in CHD events in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women-years).1 An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 [see Clinical Studies (14.3)].
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
Venous Thromboembolism
In the WHI estrogen-alone substudy, the risk of VTE (DVT and PE) was increased for women receiving daily CE (0.625 mg)-alone compared to placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women-years). The increase in VTE risk was demonstrated during the first 2 years3 [see Clinical Studies (14.3)]. Immediately discontinue estrogen-alone therapy if a VTE occurs or is suspected.
The WHI estrogen plus progestin substudy reported a statistically significant 2-fold greater rate of VTE in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women-years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted4 [see Clinical Studies (14.3)]. Immediately discontinue estrogen plus progestogen therapy if a VTE occurs or is suspected.
If feasible, discontinue estrogens at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
5.2 Malignant Neoplasms
Endometrial Cancer
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among unopposed estrogen users is about 2 to 12 times greater than in non-users and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears to be associated with prolonged use, with increased risks of 15- to 24-fold for 5 to 10 years or more, and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestogen therapy is important. Perform adequate diagnostic measures, including directed or random endometrial sampling when indicated, to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding with unknown etiology. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestogen to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
Breast Cancer
The WHI substudy of daily CE (0.625 mg)-alone provided information about breast cancer in estrogen-alone users. In the WHI estrogen-alone substudy, after an average follow-up of 7.1 years, daily CE (0.625 mg)-alone was not associated with an increased risk of invasive breast cancer (relative risk [RR] 0.80)5[see Clinical Studies (14.3)].
After a mean follow-up of 5.6 years, the WHI substudy of daily CE (0.625 mg) plus MPA (2.5 mg) reported an increased risk of invasive breast cancer in women who took daily CE plus MPA compared to placebo.
In this substudy, prior use of estrogen-alone or estrogen plus progestin therapy was reported by 26% of the women. The relative risk of invasive breast cancer was 1.24, and the absolute risk was 41 versus 33 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported prior use of hormone therapy, the relative risk of invasive breast cancer was 1.86, and the absolute risk was 46 versus 25 cases per 10,000 women-years, for CE plus MPA compared with placebo.6 Among women who reported no prior use of hormone therapy, the relative risk of invasive breast cancer was 1.09, and the absolute risk was 40 versus 36 cases per 10,000 women-years for CE plus MPA compared with placebo. In the same substudy, invasive breast cancers were larger, were more likely to be node positive, and were diagnosed at a more advanced stage in the CE (0.625 mg) plus MPA (2.5 mg) group compared with the placebo group. Metastatic disease was rare, with no apparent difference between the two groups. Other prognostic factors, such as histologic subtype, grade, and hormone receptor status did not differ between the groups6[see Clinical Studies (14.3)].
Consistent with the WHI clinical trial, observational studies have also reported an increased risk of breast cancer with estrogen plus progestin therapy, and a smaller increase in the risk for breast cancer with estrogen-alone therapy, after several years of use. One large meta-analysis of prospective cohort studies reported increased risks that were dependent upon duration of use and could last up to >10 years after discontinuation of estrogen plus progestin therapy and estrogen-alone therapy. Extension of the WHI trials also demonstrated increased breast cancer risk associated with estrogen plus progestin therapy. Observational studies also suggest that the risk of breast cancer was greater, and became apparent earlier, with estrogen plus progestin therapy as compared to estrogen-alone therapy. These studies have not generally found significant variation in the risk of breast cancer among different estrogen plus progestin combinations, doses, or routes of administration.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation. All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
Ovarian Cancer
The CE plus MPA substudy of WHI reported that estrogen plus progestin increased the risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for CE plus MPA versus placebo was 1.58 (95% CI, 0.77 to 3.24), but it was not statistically significant. The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years] vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27 to 1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
5.3 Probable Dementia
In the WHI Memory Study (WHIMS) estrogen-alone ancillary study, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo.
After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95% CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years8 [see Use in Specific Populations (8.5), and Clinical Studies (14.4)].
In the WHIMS estrogen plus progestin ancillary study of WHI, a population of 4,532 postmenopausal women 65 to 79 years was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95% CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years8 [see Use in Specific Populations (8.5), and Clinical Studies (14.4)].
When data from the two populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95% CI, 1.19 to 2.60). Since both ancillary studies were conducted in women aged 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women8 [see Use in Specific Populations (8.5), and Clinical Studies (14.4)].
5.4 Gallbladder Disease
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
5.5 Hypercalcemia
Estrogen administration may lead to severe hypercalcemia in women with breast cancer and bone metastases. Discontinue estrogens, including DOTTI, if hypercalcemia occurs, and take appropriate measures to reduce the serum calcium level.
5.6 Visual Abnormalities
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue DOTTI pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. Permanently discontinue estrogens, including DOTTI, if examination reveals papilledema or retinal vascular lesions.
5.7 Addition of a Progestogen When a Woman Has Not Had a Hysterectomy
Studies of the addition of a progestogenfor 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
There are, however, possible risks that may be associated with the use of progestogens with estrogens compared to estrogen-alone regimens. These include an increased risk of breast cancer.5.8 Elevated Blood Pressure
In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogens on blood pressure was not seen.
5.9 Exacerbation of Hypertriglyceridemia
In women with preexisting hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis. Discontinue DOTTI if pancreatitis occurs.
5.10 Hepatic Impairment and/or Past History of Cholestatic Jaundice
Estrogens may be poorly metabolized in women with hepatic impairment. Exercise caution in any woman with a history of cholestatic jaundice associated with past estrogen use or with pregnancy. In the case of recurrence of cholestatic jaundice, discontinue DOTTI.
5.11 Exacerbation of Hypothyroidism
Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Women with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T4 and T3 serum concentrations in the normal range. Women dependent on thyroid hormone replacement therapy who are also receiving estrogens may require increased doses of their thyroid replacement therapy. Monitor thyroid function in these women during treatment with DOTTI to maintain their free thyroid hormone levels in an acceptable range.
5.12 Fluid Retention
Estrogens may cause some degree of fluid retention. Monitor any woman with a condition(s) that might predispose her to fluid retention, such as cardiac or renal impairment. Discontinue estrogen-alone therapy, including DOTTI, with evidence of medically concerning fluid retention.
5.13 Hypocalcemia
Estrogen induced hypocalcemia may occur in women with hypoparathyroidism. Consider whether the benefits of estrogen therapy, including DOTTI, outweigh the risks in such women.
5.14 Exacerbation of Endometriosis
A few cases of malignant transformation of residual endometrial implants have been reported in women treated post-hysterectomy with estrogen-alone therapy. Consider the addition of progestogen therapy for women known to have residual endometriosis post-hysterectomy.
5.15 Severe Anaphylactic/Anaphylactoid Reactions and Angioedema
A few cases of anaphylactic/anaphylactoid reactions are reported in the postmarketing use of DOTTI. Involvement of skin (hives, pruritus, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) are noted.
Angioedema involving eye/eyelid, face, larynx, pharynx, tongue and extremity (hands, legs, ankles, and fingers) with or without urticaria requiring medical intervention are reported in the postmarketing use of DOTTI. Angioedema involving the tongue, glottis, or larynx, may result in airway obstruction. Do not give DOTTI to any woman who develops angioedema during treatment with DOTTI.
Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema.
Consider whether the benefits of estrogen therapy outweigh the risks in such women.
5.16 Exacerbation of Other Conditions
Estrogen therapy, including DOTTI, may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraines, porphyria, systemic lupus erythematosus, and hepatic hemangiomas. Consider whether the benefits of estrogen therapy outweigh the risks in such women.
5.17 Laboratory Tests
Serum follicle-stimulating hormone (FSH) and estradiol levels have not been shown to be useful in the management of moderate to severe vasomotor symptoms and moderate to severe symptoms of vulvar and vaginal atrophy.
Laboratory parameters may be useful in guiding dosage for the treatment of hypoestrogenism due to hypogonadism, castration and primary ovarian failure.
5.18 Drug-Laboratory Test Interactions
- Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex; and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III; decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
- Increased thyroid-binding globulin (TBG) leading to increased circulating total thyroid hormone levels, as measured by protein-bound iodine (PBI), T4 levels (by column or by radioimmunoassay) or T3 levels by radioimmunoassay. T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 and free T3 concentrations are unaltered. Women on thyroid replacement therapy may require higher doses of thyroid hormone.
- Other binding proteins may be elevated in serum, for example, corticosteroid-binding globulin (CBG), sex hormone-binding globulin (SHBG), leading to increased total circulating corticosteroids and sex steroids, respectively. Free hormone concentrations, such as testosterone and estradiol, may be decreased. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-antitrypsin, ceruloplasmin).
- Increased plasma high-density lipoprotein (HDL) and HDL2 cholesterol subfraction concentrations, reduced low-density lipoprotein (LDL) cholesterol concentration, and increased triglycerides levels.
- Impaired glucose tolerance.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in labeling:
- Cardiovascular Disorders [see Boxed Warning, Warnings and Precautions (5.1)]
- Malignant Neoplasms [see Boxed Warning, Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
There were no clinical trials conducted with DOTTI. DOTTI is bioequivalent to the original formulation of estradiol transdermal system. The following adverse reactions have been reported with the original formulation of estradiol transdermal system therapy:Table 1. Summary of Most Frequently Reported Adverse Reactions Regardless of Relationship Reported at a Frequency ≥5 Percent
Estradiol
0.025 mg/day†
(N=47)
N (%)Estradiol
0.0375 mg/day†
(N=130)
N (%)Estradiol
0.05 mg/day†
(N=103)
N (%)Estradiol
0.075 mg/day†
(N=46)
N (%)Estradiol
0.1 mg/day†
(N=132)
N (%)Placebo
(N=157)
N (%)Gastrointestinal disorders
Constipation
2 (4.3)
5 (3.8)
4 (3.9)
3 (6.5)
2 (1.5)
4 (2.5)
Dyspepsia
4 (8.5)
12 (9.2)
3 (2.9)
2 (4.3)
0
10 (6.4)
Nausea
2 (4.3)
8 (6.2)
4 (3.9)
0
7 (5.3)
5 (3.2)
General disorders and administration site conditions***
Influenza-like illness
3 (6.4)
6 (4.6)
8 (7.8)
0
3 (2.3)
10 (6.4)
Pain NOS*
0
8 (6.2)
0
2 (4.3)
7 (5.3)
7 (4.5)
Infections and infestations
Influenza
4 (8.5)
4 (3.1)
6 (5.8)
0
10 (7.6)
14 (8.9)
Nasopharyngitis
3 (6.4)
16 (12.3)
10 (9.7)
9 (19.6)
11 (8.3)
24 (15.3)
Sinusitis NOS*
4 (8.5)
17 (13.1)
13 (12.6)
3 (6.5)
7 (5.3)
16 (10.2)
Upper respiratory tract infection NOS*
3 (6.4)
8 (6.2)
11 (10.7)
4 (8.7)
6 (4.5)
9 (5.7)
Investigations
Weight increased
4 (8.5)
5 (3.8)
2 (1.9)
2 (4.3)
0
3 (1.9)
Musculoskeletal and connective tissue disorders
Arthralgia
0
11 (8.5)
4 (3.9)
2 (4.3)
5 (3.8)
9 (5.7)
Back pain
4 (8.5)
10 (7.7)
9 (8.7)
4 (8.7)
14 (10.6)
10 (6.4)
Neck pain
3 (6.4)
4 (3.1)
4 (3.9)
0
6 (4.5)
2 (1.3)
Pain in limb
0
10 (7.7)
7 (6.8)
2 (4.3)
6 (4.5)
9 (5.7)
Nervous system disorders
Headache NOS*
7 (14.9)
35 (26.9)
32 (31.1)
23 (50.0)
34 (25.8)
37 (23.6)
Sinus headache
0
12 (9.2)
5 (4.9)
5 (10.9)
2 (1.5)
8 (5.1)
Psychiatric disorders
Anxiety NEC**
3 (6.4)
5 (3.8)
0
0
2 (1.5)
4 (2.5)
Depression
5 (10.6)
4 (3.1)
7 (6.8)
0
4 (3.0)
6 (3.8)
Insomnia
3 (6.4)
6 (4.6)
4 (3.9)
2 (4.3)
2 (1.5)
9 (5.7)
Reproductive system and breast disorders
Breast tenderness
8 (17.0)
10 (7.7)
8 (7.8)
3 (6.5)
17 (12.9)
0
Dysmenorrhea
0
0
0
3 (6.5)
0
0
Intermenstrual bleeding
3 (6.4)
9 (6.9)
6 (5.8)
0
14 (10.6)
7 (4.5)
Respiratory, thoracic and mediastinal disorders
Sinus congestion
0
4 (3.1)
3 (2.9)
3 (6.5)
6 (4.5)
7 (4.5)
Vascular disorders
Hot flushes NOS*
3 (6.4)
0
3 (2.9)
0
0
6 (3.8)
Hypertension NOS*
2 (4.3)
0
3 (2.9)
0
0
2 (1.3)
† Represents milligrams of estradiol delivered daily by each system.
* NOS represents not otherwise specified.
** NEC represents not elsewhere classified.
*** Application site erythema and application site irritation were observed in a small number of patients (3.2% or less of patients across treatment groups).
6.2 Post-marketing Experience
The following additional adverse reactions have been identified during post-approval use of DOTTI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Genitourinary System
Vaginal hemorrhage and abnormal withdrawal bleeding or flow, breakthrough bleeding, spotting, uterine leiomyomata, vaginitis, vaginal discharge, ovarian cancer, endometrial hyperplasia, dysmenorrhea.
Breast
Enlargement, pain, nipple discharge, fibrocystic breast changes, breast cancer.
Cardiovascular
Deep venous thrombosis, pulmonary embolism, thrombophlebitis.
Gastrointestinal
Nausea, vomiting, abdominal cramps, bloating, cholelithiasis, liver function tests abnormal, diarrhea.
Skin
Application site reactions include localized bleeding, bruising, burning, discomfort, dryness, eczema, edema, erythema, erythema multiforme, erythema nodosum, inflammation, irritation, pain, papules and vesicles. Other skin reactions include paresthesia, skin discoloration, skin pigmentation, urticaria, swelling, loss of scalp hair, hirsutism, pruritus, and rash.
Eyes
Intolerance to contact lenses.
Central Nervous System
Migraine, dizziness, chorea, nervousness, affect liability, irritability.
Miscellaneous
Decrease in weight, reduced carbohydrate tolerance, edema, arthralgias, leg cramps, changes in libido, purpura, hypersensitivity, anaphylactic reaction, anaphylactoid reaction, angioedema.
-
7 DRUG INTERACTIONS
In vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism. Inducers of CYP3A4 such as St. John’s wort (Hypericum perforatum) preparations, phenobarbital, carbamazepine and rifampin may reduce plasma concentrations of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. Inhibitors of CYP3A4 such as erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir, and grapefruit juice may increase plasma concentrations of estrogens and may result in adverse reactions.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
DOTTI is not indicated for use in pregnancy. There are no data with the use of DOTTI in pregnant women; however, epidemiologic studies and meta-analyses have not found an increased risk of genital or nongenital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to combined hormonal contraceptives (estrogen and progestins) before conception or during early pregnancy.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
Estrogens are present in human milk and can reduce milk production in breast-feeding women. This reduction can occur at any time but is less likely to occur once breast-feeding is well-established.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for DOTTI and any potential adverse effects on the breastfed child from DOTTI or from the underlying maternal condition.
8.4 Pediatric Use
DOTTI is not indicated for use in pediatric patients. Clinical studies have not been conducted in the pediatric population.
If estrogen is administered to patients whose bone growth is not complete, periodic monitoring of bone maturation and effects on epiphyseal centers is recommended during estrogen administration.
8.5 Geriatric Use
There have not been sufficient numbers of geriatric women involved in clinical studies utilizing DOTTI to determine whether those over 65 years of age differ from younger subjects in their response to DOTTI.
The Women’s Health Initiative Studies
In the WHI estrogen-alone substudy (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age [see Warnings and Precautions (5.1), and Clinical Studies (14.3)].
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age [see Warnings and Precautions (5.1), and Clinical Studies (14.3)].
The Women’s Health Initiative Memory Study
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen-alone or estrogen plus progestin when compared to placebo [see Warnings and Precautions (5.3), and Clinical Studies (14.4)].
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women8[see Warnings and Precautions (5.3), and Clinical Studies (14.4)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
DOTTI (estradiol transdermal system, USP) contains estradiol, USP in a multipolymeric adhesive. The system is designed to release estradiol, USP continuously upon application to intact skin.
Five dosage strengths of DOTTI are available to provide nominal in vivo delivery rates of 0.025, 0.0375, 0.05, 0.075, or 0.1 mg of estradiol, USP per day via the skin. Each corresponding system has an active surface area of 1.89, 2.83, 3.78, 5.66, or 7.55 cm2 and contains 0.314, 0.470, 0.627, 0.940, or 1.253 mg of estradiol USP, respectively. The composition of the systems per unit area is identical.
Estradiol, USP is a white to practically white powder, chemically described as estra-1,3,5 (10)- triene-3,17β-diol.
The structural formula is:
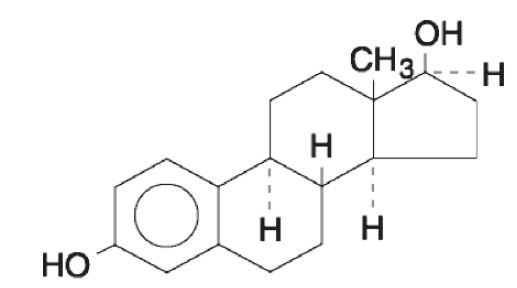
The molecular formula of estradiol, USP is C18H2402. The molecular weight is 272.39 g/mol.
DOTTI is comprised of 3 layers. Proceeding from the visible surface toward the surface attached to the skin, these layers are (1) polyester and ethylene vinyl acetate copolymer film (2) an adhesive formulation containing estradiol USP, acrylic adhesive, silicone adhesive, oleyl alcohol, NF, povidone, USP and dipropylene glycol, and (3) a polyester release liner which is attached to the adhesive surface and must be removed before the system can be used.
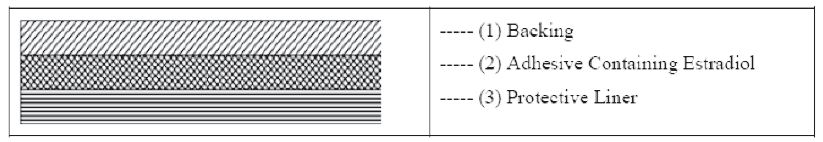
The active component of the system is estradiol, USP. The remaining components of the system are pharmacologically inactive.
FDA approved acceptance criteria for dissolution test specifications differ from USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol, at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone in the peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, 2 estrogen receptors have been identified. These vary in proportion from tissue to tissue.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH) through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.
12.2 Pharmacodynamics
Generally, a serum estrogen concentration does not predict an individual woman’s therapeutic response to DOTTI nor her risk for adverse outcomes. Likewise, exposure comparisons across different estrogen products to infer efficacy or safety for the individual woman may not be valid.
12.3 Pharmacokinetics
Absorption
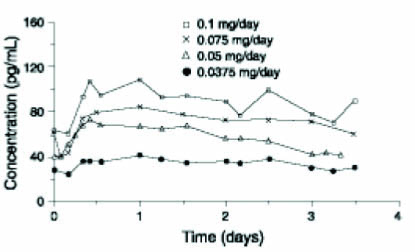
In a multiple-dose study consisting of 3 consecutive system applications of the original formulation of estradiol transdermal system which was conducted in 17 healthy, postmenopausal women, blood levels of estradiol and estrone were compared following application of these units to sites on the abdomen and buttocks in a crossover fashion. Systems that deliver nominal estradiol doses of approximately 0.0375 mg per day and 0.1 mg per day were applied to abdominal application sites while the 0.1 mg per day doses were also applied to sites on the buttocks. These systems increased estradiol levels above baseline within 4 hours and maintained respective mean levels of 25 and 79 pg/mL above baseline following application to the abdomen; slightly higher mean levels of 88 pg/mL above baseline were observed following application to the buttocks. At the same time, increases in estrone plasma concentrations averaged about 12 and 50 pg/mL, respectively, following application to the abdomen and 61 pg/mL for the buttocks. While plasma concentrations of estradiol and estrone remained slightly above baseline at 12 hours following removal of the systems in this study, results from another study show these levels to return to baseline values within 24 hours following removal of the systems.
Figure 1 illustrates the mean plasma concentrations of estradiol at steady-state during application of these patches at 4 different dosages.
Figure 1. Steady-State Estradiol Plasma Concentrations for Systems Applied to the Abdomen Nonbaseline-corrected Levels
The corresponding pharmacokinetic parameters are summarized in Table 2.
Table 2. Steady-State Estradiol Pharmacokinetic Parameters for Systems Applied to the Abdomen (mean ± standard deviation) Nonbaseline-corrected Data*
Dosage
(mg/day)Cmax†
(pg/mL)Cavg‡
(pg/mL)Cmin (84 hr)§
(pg/mL)0.0375
46 ± 16
34 ± 10
30 ±10
0.05
83 ± 41
57 ± 23#
41 ± 11#
0.075
99 ± 35
72 ± 24
60 ± 24
0.1
133 ± 51
89 ± 38
90 ± 44
0.1¶
145 ± 71
104 ± 52
85 ± 47
*Mean baseline estradiol concentration =11.7 pg/mL.
†Peak plasma concentration.
‡Average plasma concentration.
§Minimum plasma concentration at 84 hr.
#Measured over 80 hr.
¶Applied to the buttocks.
DOTTI (estradiol transdermal system), the revised formulation with smaller system sizes, was shown to be bioequivalent to the original formulation of estradiol transdermal system, used in the clinical trials.
Distribution
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estrogens circulate in the blood largely bound to sex hormone-binding globulin (SHBG) and albumin.
Metabolism
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver by Cytochrome 450 isoforms CYP1A2 and CYP3A4. Estradiol undergoes further metabolism to sulfate and glucuronide conjugates. Estradiol and its metabolites are glucuronidated by UGT1A1 and UGT2B7. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is a major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the intestine followed by reabsorption. In postmenopausal women a significant portion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
Excretion
Estradiol, estrone and estriol are excreted in the urine along with glucuronide and sulfate conjugates. The half-life values calculated after dosing with DOTTI ranged from 5.9 to 7.7 hours. After removal of the transdermal systems, serum concentrations of estradiol and estrone returned to baseline levels within 24 hours.
Adhesion
Based on combined data from 3 short-term clinical trials consisting of 471 observations, 85% of DOTTI adhered completely to the skin over the 3.5-day wear period. Three percent (3%) of the systems detached and were reapplied or replaced during the 3.5-day wear period. Approximately 80% of the transdermal systems evaluated in these studies were DOTTI 0.05 mg per day.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Effects on Vasomotor Symptoms in Postmenopausal Women
In a pharmacokinetic study, DOTTI was shown to be bioequivalent to the original estradiol formulation. In 2 controlled clinical trials with the original estradiol formulation, of 356 women, the 0.075 and 0.1 mg doses were superior to placebo in relieving vasomotor symptoms at Week 4, and maintained efficacy through Weeks 8 and 12 of treatment. In this original study, the 0.0375 and 0.05 mg doses, however, did not differ from placebo until approximately Week 6, therefore, an additional 12-week, placebo-controlled study in 255 women was performed with the original estradiol formulation to establish the efficacy of the lowest dose of 0.0375 mg. The baseline mean daily number of hot flushes in these 255 women was 11.5. Results at Weeks 4, 8, and 12 of treatment are shown in Figure 2.
Figure 2. Mean (SD) Change from Baseline in Mean Daily Number of Flushes for Estradiol 0.0375 mg Versus Placebo in a 12-week Trial
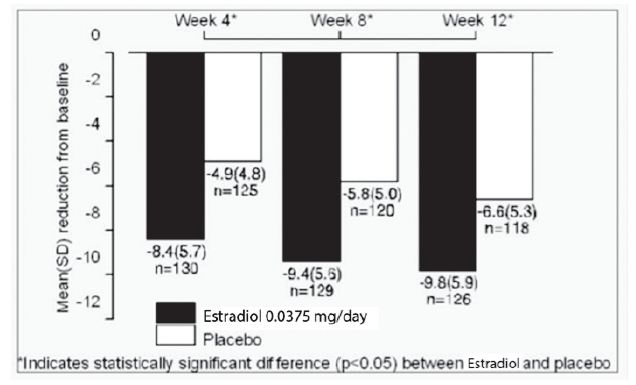
The 0.0375 mg dose was superior to placebo in reducing both the frequency and severity of vasomotor symptoms at Week 4 and maintained efficacy through Weeks 8 and 12 of treatment. All doses of the original estradiol formulation (0.0375 mg, 0.05 mg, 0.075 mg, and 0.1 mg) are effective for the control of vasomotor symptoms.
14.2 Effects on Bone Mineral Density in Postmenopausal Women
Efficacy and safety of the original estradiol formulation in the prevention of postmenopausal osteoporosis have been studied in a 2-year, double-blind, randomized, placebo-controlled, parallel-group study. A total of 261 hysterectomized (161) and non-hysterectomized (100), surgically or naturally menopausal women (within 5 years of menopause), with no evidence of osteoporosis (lumbar spine bone mineral density within 2 standard deviations of average peak bone mass, i.e., at least 0.827 g/cm2) were enrolled in this study; 194 women were randomized to 1 of the 4 doses of the original estradiol formulation (0.1, 0.05, 0.0375, or 0.025 mg/day) and 67 patients to placebo. Over 2 years, study systems were applied to the buttock or the abdomen twice a week. Non-hysterectomized women received oral medroxyprogesterone acetate (2.5 mg/day) throughout the study.
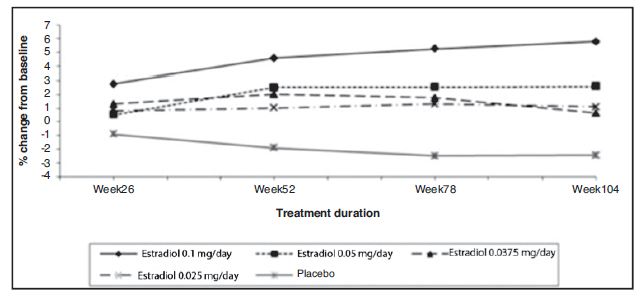
The study population comprised naturally (82%) or surgically (18%) menopausal, hysterectomized (61%) or non-hysterectomized (39%) women with a mean age of 52 years (range 27 to 62 years); the mean duration of menopause was 31.7 months (range 2 to 72 months). Two hundred thirty-two (89%) of randomized women (173 on active drug, 59 on placebo) contributed data to the analysis of percent change from baseline in bone mineral density (BMD) of the AP lumbar spine, the primary efficacy variable. Women were given supplemental dietary calcium (1000 mg elemental calcium/day) but no supplemental vitamin D. There was an increase in BMD of the AP lumbar spine in all the original estradiol formulation dose groups; in contrast to this, a decrease in AP lumbar spine BMD was observed in placebo patients. All estradiol doses were significantly superior to placebo (p<0.05) at all time points with the exception of estradiol 0.05 mg/day at 6 months. The highest dose of estradiol was superior to the 3 lower doses. There were no statistically significant differences in pairwise comparisons among the 3 lower doses (See Figure 3).
Figure 3. Bone Mineral Density-AP Lumbar Spine Least Squares Means of Percentage Change from Baseline All Randomized Patients with at Least One Post-baseline Assessment Available with Last Post-baseline Observation Carried Forward
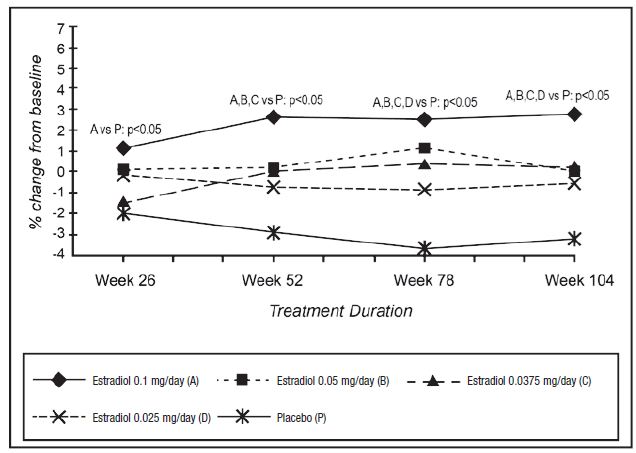
Analysis of percent change from baseline in femoral neck BMD, a secondary efficacy outcome variable, showed qualitatively similar results; all doses of the original estradiol formulation were significantly superior to placebo (p<0.05) at 24 months. The highest estradiol dose was superior to placebo at all time points. A mixture of significant and nonsignificant results were obtained for the lower dose groups at earlier time points. The highest estradiol dose was superior to the 3 lower doses, and there were no significant differences among the 3 lower doses at this skeletal site (See Figure 4).
Figure 4. Bone Mineral Density-Femoral Neck Least Squares Means of Percentage Change from Baseline All Randomized Patients with at Least One Post-baseline Assessment Available with Last Post-baseline Observation Carried Forward
The mean serum osteocalcin (a marker of bone formation) and urinary excretion of cross-link N-telopeptides of Type 1 collagen (a marker of bone resorption) decreased numerically in most of the active treatment groups relative to baseline. However, the decreases in both markers were inconsistent across treatment groups and the differences between active treatment groups and placebo were not statistically significant.
14.3 Women’s Health Initiative Studies
The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with the MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of CHD (defined as nonfatal MI, silent MI, and CHD death), with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other causes. These substudies did not evaluate the effects of CE-alone or CE plus MPA on menopausal symptoms.
WHI Estrogen-Alone Substudy
The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints. Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79; 75.3% White, 15.1% Black, 6.1% Hispanic, 3.6% Other) after an average follow-up of 7.1 years, are presented in Table 3.
Table 3. Relative and Absolute Risk Seen in the Estrogen-Alone Substudy of WHIa
Event
Relative Risk
CE vs. Placebo
(95% nCIb)CE
n=5,310Placebo
n=5,429
Absolute Risk per 10,000
Women-YearsCHD eventsc
0.95 (0.78 to 1.16)
54
57
Non-fatal MIc
0.91 (0.73 to 1.14)
40
43
CHD deathc
1.01 (0.71 to 1.43)
16
16
All strokesc
1.33 (1.05 to 1.68)
45
33
Ischemic strokec
1.55 (1.19 to 2.01)
38
25
Deep vein thrombosisc,d
1.47 (1.06 to 2.06)
23
15
Pulmonary embolismc
1.37 (0.90 to 2.07)
14
10
Invasive breast cancerc
0.80 (0.62 to 1.04)
28
34
Colorectal cancere
1.08 (0.75 to 1.55)
17
16
Hip fracturec
0.65 (0.45 to 0.94)
12
19
Vertebral fracturesc,d
0.64 (0.44 to 0.93)
11
18
Lower arm/wrist fracturesc,d
0.58 (0.47 to 0.72)
35
59
Total fracturesc,d
0.71 (0.64 to 0.80)
144
197
Death due to other causese,f
1.08 (0.88 to 1.32)
53
50
Overall mortalityc,d
1.04 (0.88 to 1.22)
79
75
Global Indexg
1.02 (0.92 to 1.13)
206
201
a Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi.
b Nominal confidence intervals unadjusted for multiple looks and multiple comparisons.
c Results are based on centrally adjudicated data for an average follow-up of 7.1 years.
d Not included in “global index”.
e Results are based on an average follow-up of 6.8 years.
f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.
g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes.
For those outcomes included in the WHI “global index” that reached statistical significance, the absolute excess risk per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures.9 The absolute excess risk of events included in the “global index” was a nonsignificant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years (See Table 3).
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant differences in distribution of stroke subtype or severity, including fatal strokes, in women receiving CE-alone compared to placebo. Estrogen-alone increased the risk for ischemic stroke, and this excess risk was present in all subgroups of women examined10 (See Table 3).
Timing of the initiation of estrogen-alone therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age a nonsignificant trend toward reduced risk for CHD [hazard ratio (HR) 0.63 (95% CI, 0.36 to 1.09)] and overall mortality [HR 0.71 (95% CI, 0.46 to 1.11)].
WHI Estrogen Plus Progestin Substudy
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the “global index”. The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women-years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reduction per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the CE plus MPA substudy, which included 16,608 women (average 63 years of age, range 50 to 79, 83.9% White, 6.8% Black, 5.4% Hispanic, 3.9% Other) are presented in Table 4. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
Table 4. Relative and Absolute Risk Seen in the Estrogen Plus Progestin Substudy of WHI at an Average of 5.6 Yearsa,b
Event
Relative Risk
CE/MPA vs. Placebo
(95% nCIc)CE/MPA
n=8,506Placebo
n=8,102
Absolute Risk per 10,000
Women-YearsCHD events
1.23 (0.99 to 1.53)
41
34
Non-fatal MI
1.28 (1.00 to 1.63)
31
25
CHD death
1.10 (0.70 to 1.75)
8
8
All strokes
1.31 (1.03 to 1.68)
33
25
Ischemic Stroke
1.44 (1.09 to 1.90)
26
18
Deep vein thrombosisd
1.95 (1.43 to 2.67)
26
13
Pulmonary embolism
2.13 (1.45 to 3.11)
18
8
Invasive breast cancere
1.24 (1.01 to 1.54)
41
33
Colorectal cancer
0.61 (0.42 to 0.87)
10
16
Endometrial cancerd
0.81 (0.48 to 1.36)
6
7
Cervical cancerd
1.44 (0.47 to 4.42)
2
1
Hip fracture
0.67 (0.47 to 0.96)
11
16
Vertebral fracturesd
0.65 (0.46 to 0.92)
11
17
Lower arm/wrist fracturesd
0.71 (0.59 to 0.85)
44
62
Total fracturesd
0.76 (0.69 to 0.83)
152
199
Overall mortalityf
1.00 (0.83 to 1.19)
52
52
Global Indexg
1.13 (1.02 to 1.25)
184
165
a Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi.
b Results are based on centrally adjudicated data.
c Nominal confidence intervals unadjusted for multiple looks and multiple comparisons.
d Not included in “global index”.
e Includes metastatic and non-metastatic breast cancer, with the exception of in situ breast cancer.
f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.
g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes.
Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a non-significant trend toward reduced risk for overall mortality [HR 0.69 (95% CI, 0.44 to 1.07)].
14.4 Women’s Health Initiative Memory Study
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age and older (45% were age 65 to 69 years of age; 36% were 70 to 74 years of age; 19% were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95% CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in this study included Alzheimer’s disease (AD), vascular dementia (VaD), and mixed types (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women [see Warnings and Precautions (5.3)and Use in Specific Populations (8.5)].
The WHIMS estrogen plus progestin ancillary study enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47% were age 65 to 69 years of age; 35% were 70 to 74 years of age; 18% were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA was 2.05 (95% CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 per 10,000 women-years. Probable dementia as defined in this study included AD, VaD, and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women [see Warnings and Precautions (5.3)and Use in Specific Populations (8.5)].
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95% CI, 1.19 to 2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women [see Warnings and Precautions (5.3) and Use in Specific Populations (8.5)].
-
15 REFERENCES
- Rossouw JE, et al. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause. JAMA. 2007; 297:1465-1477.
- Hsia J, et al. Conjugated Equine Estrogens and Coronary Heart Disease. Arch Int Med. 2006; 166:357-365.
- Curb JD, et al. Venous Thrombosis and Conjugated Equine Estrogen in Women Without a Uterus. Arch Int Med. 2006; 166:772-780.
- Cushman M, et al. Estrogen Plus Progestin and Risk of Venous Thrombosis. JAMA. 2004; 292:1573-1580.
- Stefanick ML, et al. Effects of Conjugated Equine Estrogens on Breast Cancer and Mammography Screening in Postmenopausal Women With Hysterectomy. JAMA. 2006; 295:1647-1657.
- Chlebowski RT, et al. Influence of Estrogen Plus Progestin on Breast Cancer and Mammography in Healthy Postmenopausal Women. JAMA. 2003; 289:3234-3253.
- Anderson GL, et al. Effects of Estrogen Plus Progestin on Gynecologic Cancers and Associated Diagnostic Procedures. JAMA. 2003; 290:1739-1748.
- Shumaker SA, et al. Conjugated Equine Estrogens and Incidence of Probable Dementia and Mild Cognitive Impairment in Postmenopausal Women. JAMA. 2004; 291:2947-2958.
- Jackson RD, et al. Effects of Conjugated Equine Estrogen on Risk of Fractures and BMD in Postmenopausal Women With Hysterectomy: Results From the Women's Health Initiative Randomized Trial. J Bone Miner Res. 2006; 21:817-828.
- Hendrix SL, et al. Effects of Conjugated Equine Estrogen on Stroke in the Women's Health Initiative. Circulation. 2006; 113:2425-2434.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
DOTTI (estradiol transdermal system, USP), 0.075 mg per day- each 5.66 cm2 system contains 0.940 mg of estradiol USP for nominal* delivery of 0.075 mg of estradiol, USP per day.
- Patient Calendar Pack of 8 Systems………………………………….NDC: 72162-2035-2
16.2 Storage and Handling
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Do not store unpouched. Apply immediately upon removal from the protective pouch.
Used transdermal systems still contain active hormone. To discard, fold the sticky side of the transdermal system together, place it in a sturdy child-proof container, and place this container in the trash. Used transdermal systems should not be flushed in the toilet.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504 -
17 PATIENT COUNSELING INFORMATION
Advise women to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
Vaginal Bleeding
Inform postmenopausal women to report any vaginal bleeding to their healthcare providers as soon as possible [see Warnings and Precautions (5.2)].
Possible Serious Adverse Reactions with Estrogen-Alone Therapy
Inform postmenopausal women of possible serious adverse reactions of estrogen-alone therapy including Cardiovascular Disorders, Malignant Neoplasms, and Probable Dementia [see Warnings and Precautions (5.1, 5.2, 5.3)].
Possible Common Adverse Reactions with Estrogen-Alone Therapy
Inform postmenopausal women of possible less serious but common adverse reactions of estrogen-alone therapy such as headache, breast pain and tenderness, nausea and vomiting.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807Rev. 05-2024-03
-
PATIENT INFORMATION
DOTTI (dahʹ tee)
(estradiol transdermal system)Read this Patient Information before you start using DOTTI and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your menopausal symptoms or your treatment.
What is the most important information I should know about DOTTI (an estrogen hormone)?
- Using estrogen-alone increases your chance of getting cancer of the uterus (womb).
- Report any unusual vaginal bleeding right away while you are using DOTTI. Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
- Do not use estrogen-alone to prevent heart disease, heart attacks, strokes, or dementia (decline in brain function).
- Using estrogen-alone may increase your chances of getting strokes or blood clots.
- Using estrogen-alone may increase your chance of getting dementia, based on a study of women 65 years of age and older.
- Do not use estrogens with progestogens to prevent heart disease, heart attacks, strokes, or dementia.
- Using estrogens with progestogens may increase your chances of getting heart attacks, strokes, breast cancer, or blood clots.
- Using estrogens with progestogens may increase your chance of getting dementia, based on a study of women 65 years of age and older.
- Only one estrogen-alone product and dose have been shown to increase your chances of getting strokes, blood clots, and dementia. Only one estrogen with progestogen product and dose have been shown to increase your chances of getting heart attacks, strokes, breast cancer, blood clots, and dementia.
Because other products and doses have not been studied in the same way, it is not known how the use of DOTTI will affect your chances of these conditions. You and your healthcare provider should talk regularly about whether you still need treatment with DOTTI.
What is DOTTI?
DOTTI is a prescription medicine patch (transdermal system) that contains the estrogen hormone estradiol. When applied to the skin, estradiol is absorbed through the skin into the bloodstream.What is DOTTI used for?
DOTTI is used after menopause to:- Reduce moderate to severe hot flashes
Estrogens are hormones made by a woman’s ovaries. The ovaries normally stop making estrogens when a woman is between 45 and 55 years old. This drop in body estrogen levels causes the “change of life” or menopause (the end of monthly menstrual periods). Sometimes, both ovaries are removed during an operation before natural menopause takes place. The sudden drop in estrogen levels causes “surgical menopause.”
When estrogen levels begin dropping, some women develop very uncomfortable symptoms, such as feelings of warmth in the face, neck, and chest or sudden intense feelings of heat and sweating (“hot flashes” or “hot flushes”). In some women the symptoms are mild, and they will not need to use estrogens. In other women, symptoms can be more severe.
- Treat moderate to severe menopausal changes in and around the vagina
You and your healthcare provider should talk regularly about whether you still need treatment with DOTTI to control these problems. If you use DOTTI only to treat your menopausal changes in and around your vagina, talk with your healthcare provider about whether a topical vaginal product would be better for you.
- Treat certain conditions in women before menopause if their ovaries do not produce enough estrogens naturally
- Help reduce your chances of getting osteoporosis (thin weak bones)
Osteoporosis from menopause is a thinning of the bones that makes them weaker and easier to break. If you use DOTTI to prevent osteoporosis due to menopause, talk with your healthcare provider about whether a different treatment or medicine without estrogens might be better for you.
You and your healthcare provider should talk regularly about whether you should continue treatment with DOTTI.Who should not use DOTTI? Do not start using DOTTI if you:
-
have unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
- have been diagnosed with a bleeding disorder
-
currently have or have had certain cancers
Estrogens may increase the chances of getting certain types of cancers, including cancer of the breast or uterus (womb). If you have or have had cancer, talk with your healthcare provider about whether you should use DOTTI.
- had a stroke or heart attack
- currently have or have had blood clots
- currently have or have had liver problems
- are allergic to DOTTI or any of the ingredients in it
See the list of ingredients in DOTTI at the end of this leaflet.
Before you use DOTTI, tell your healthcare provider about all of your medical conditions, including if you:
-
have any unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
-
have any other medical conditions that may become worse while you are using DOTTI
Your healthcare provider may need to check you more carefully if you have certain conditions such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis, lupus, angioedema (swelling of face and tongue); problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood.
-
are going to have surgery or will be on bed rest
Your healthcare provider will let you know if you need to stop using DOTTI.
-
are pregnant or think you may be pregnant.
DOTTI is not for pregnant women.
- are breastfeeding
The hormone in DOTTI can pass into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how DOTTI works. DOTTI may also affect how your other medicines work. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get new medicine.
How should I use DOTTI?
For detailed instructions, see the step-by-step instructions for using DOTTI at the end of this Patient Information.- Use DOTTI exactly as your healthcare provider tells you to use it.
- DOTTI is for skin use only.
- Change your DOTTI patch 2 times a week or every 3 to 4 days.
- Apply your DOTTI patch to a clean, dry area on your lower abdomen. This area must be clean, dry, and free of powder, oil or lotion for your patch to stick to your skin.
- Apply your DOTTI patch to a different area of your abdomen each time. Do not use the same application site 2 times in the same week.
- Do not apply DOTTI to your breasts.
- If you forget to apply a new DOTTI patch, apply a new patch as soon as possible.
- You and your healthcare provider should talk regularly (every 3 to 6 months) about your dose and whether you still need treatment with DOTTI.
How to change DOTTI
- When changing the patch, peel off the used patch slowly from the skin.
- After removal of DOTTI if any adhesive residue remains on your skin, allow the area to dry for 15 minutes. Then, gently rub the area with oil or lotion to remove the adhesive from your skin.
- Apply the new patch to a different area of your lower abdomen. This area must be clean, dry, cool and free of powder, oil, or lotion.
What are the possible side effects of DOTTI?
Side effects are grouped by how serious they are and how often they happen when you are treated.
Serious, but less common side effects include:- heart attack
- high blood pressure
- stroke
- high levels of fat (triglyceride) in your blood
- blood clots
- liver problems
- breast cancer
- changes in your thyroid hormone levels
- cancer of the lining of the uterus (womb)
- fluid retention
- cancer of the ovary
- cancer changes of endometriosis
- dementia
- enlargement of benign tumors of the uterus (“fibroids”)
- high or low blood calcium
- worsening of swelling of face and tongue (angioedema) in women with a history of angioedema
- gallbladder disease
- visual abnormalities
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- new breast lumps
- unusual vaginal bleeding
- changes in vision or speech
- sudden new severe headaches
- severe pains in your chest or legs with or without shortness of breath, weakness and fatigue
- swelling of face and tongue with or without red, itchy bumps
Common side effects of DOTTI include:
- headache
- nausea and vomiting
- breast pain
- hair loss
- irregular vaginal bleeding or spotting
- fluid retention
- painful periods
- vaginal yeast infection
- stomach or abdominal cramps, bloating
- redness and/or irritation at patch placement site
These are not all the possible side effects of DOTTI. For more information, ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effects that bother you or do not go away.
You may report side effects to FDA at 1-800-FDA-1088. You may report side effects to Amneal Pharmaceuticals (1-877-835-5472).
What can I do to lower my chances of getting a serious side effect with DOTTI?
- Talk with your healthcare provider regularly about whether you should continue using DOTTI.
- If you have a uterus, talk to your healthcare provider about whether the addition of a progestogen is right for you. In general, the addition of a progestogen is recommended for a woman with a uterus to reduce the chance of getting cancer of the uterus (womb).
- See your healthcare provider right away if you get vaginal bleeding while using DOTTI.
- Have a pelvic exam, breast exam and mammogram (breast X-ray) every year unless your healthcare provider tells you something else. If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram, you may need to have breast exams more often.
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have higher chances for getting heart disease.
Ask your healthcare provider for ways to lower your chances for getting heart disease.
How should I store and throw away used DOTTI patches?
- Store DOTTI at room temperature 68° to 77°F (20° to 25°C)
- Do not store DOTTI patches outside of their pouches. Apply immediately upon removal from the protective pouch.
- Used patches still contain estrogen. To throw away the patch, fold the sticky side of the patch together, place it in a sturdy child-proof container, and place this container in the trash. Used patches should not be flushed in the toilet.
Keep DOTTI and all medicines out of the reach of children.
General information about safe and effective use of DOTTI
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use DOTTI for conditions for which it was not prescribed. Do not give DOTTI to other people, even if they have the same symptoms you have. It may harm them.You can ask your healthcare provider or pharmacist for information about DOTTI that is written for health professionals. For more information, go to www.amneal.com or call the toll-free number Amneal Pharmaceuticals (1-877-835-5472).
What are the ingredients in DOTTI?
Active ingredient: estradiol, USP
Inactive ingredients: a polyester and ethylene vinyl acetate copolymer film, acrylic and silicone adhesives, oleyl alcohol, NF, povidone, USP, dipropylene glycol and a polyester release liner.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807Rev. 05-2024-03
This Patient Information has been approved by the U.S. Food and Drug Administration.
-
INSTRUCTIONS FOR USE
DOTTI (dahʹ tee)
(estradiol transdermal system)Read this Instructions for Use before you start using DOTTI and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your menopausal symptoms or your treatment.
1. Determine Your Schedule for Your Twice-a-Week Application
- Decide upon which 2 days you will change your patch.
- Your DOTTI (estradiol transdermal system) individual carton contains a calendar card printed on its inner flap. Mark the 2-day schedule you plan to follow on your carton’s inner flap.
- Be consistent.
- If you forget to change your patch on the correct date, apply a new one as soon as you remember.
- No matter what day this happens, stick to the schedule you have marked on the inner flap of your carton (your calendar card).
2. Where to Apply DOTTI
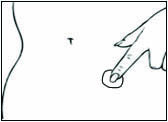
- Apply patch to a dry area of the skin of the trunk of the body, including the lower abdomen, or buttocks. Avoid the waistline, since clothing may cause the patch to rub off.
- Do not apply patch to breasts.
- When changing your patch, based on your twice-a-week schedule, apply your new patch to a different site. Do not apply a new patch to that same area for at least 1 week.
3. Before You Apply DOTTI

Make sure your skin is:
- Clean (freshly washed), dry and cool.
- Free of any powder, oil, moisturizer or lotion.
- Free of cuts or irritations (rashes or other skin problems).
4. How to Apply DOTTI
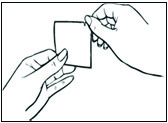
- Each patch is individually sealed in a protective pouch.
- Tear open the pouch at the tear notch (do not use scissors).
- Remove the patch.
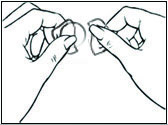
- Apply the patch immediately after removing from the pouch.
- Holding the patch with the rigid protective liner facing you, remove half of the liner, which covers the sticky surface of the patch.

- Avoid touching the sticky side of the patch with your fingers.
- Using the other half of the rigid protective liner as a handle, apply the sticky side of the patch to the selected area of the abdomen or buttocks.
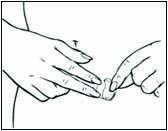
- Press the sticky side of the patch firmly into place.
- Smooth it down.
- While still holding the sticky side down, fold back the other half of the patch.
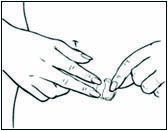
- Grasp an edge of the remaining protective liner and gently pull it off.
- Avoid touching the sticky side of the patch with your fingers.
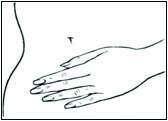
- Press the entire patch firmly into place with the palm of your hand.
- Continue to apply pressure, with the palm of your hand over the patch, for approximately 10 seconds.
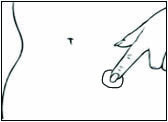
- Make sure that the patch is properly adhered to your skin.
- Go over the edges with your finger to ensure good contact around the patch.
Note:
- Showering will not cause your patch to fall off.
- If your patch falls off reapply it. If you cannot reapply the patch, apply a new patch to another area and continue to follow your original placement schedule.
- If you stop using your DOTTI patch or forget to apply a new patch as scheduled, you may have spotting, or bleeding, and recurrence of symptoms.
5. Throwing Away Your Used Patch
- When it is time to change your patch, remove the old patch before you apply a new patch.
- To throw away the patch, fold the sticky side of the patch together, place it in a sturdy child-proof container, and place the container in the trash. Used patches should not be flushed in the toilet.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807Rev. 05-2024-03
- PATIENT PACKAGE INSERT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DOTTI
estradiol patch, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72162-2035(NDC:65162-995) Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 0.075 mg in 1 d Inactive Ingredients Ingredient Name Strength DIMETHICONOL/TRIMETHYLSILOXYSILICATE CROSSPOLYMER (40/60 W/W; 1000000 PA.S) (UNII: 83D19O7250) OLEYL ALCOHOL (UNII: 172F2WN8DV) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) DIPROPYLENE GLYCOL (UNII: E107L85C40) Product Characteristics Color Score Shape ROUND Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72162-2035-2 8 in 1 CARTON 02/04/2019 1 NDC: 72162-2035-4 1 d in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211293 02/04/2019 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(72162-2035) , RELABEL(72162-2035)
Trademark Results [DOTTI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DOTTI 98192985 not registered Live/Pending |
The Imagine Group, LLC 2023-09-22 |
 DOTTI 97565516 not registered Live/Pending |
Dotti Inc. 2022-08-25 |
 DOTTI 90120704 not registered Live/Pending |
Wu Meizhen 2020-08-18 |
 DOTTI 88167435 not registered Live/Pending |
Amneal Pharmaceuticals LLC 2018-10-24 |
 DOTTI 86917096 5138878 Live/Registered |
JSJ Furniture Corporation 2016-02-23 |
 DOTTI 79338973 not registered Live/Pending |
THEREZIA PRODCOM SRL 2022-03-17 |
 DOTTI 75864925 not registered Dead/Abandoned |
DBW S.r.L. 1999-12-06 |
 DOTTI 74244531 1774276 Live/Registered |
NOVO FASHION LIMITED 1992-02-10 |
 DOTTI 73233311 1155870 Dead/Cancelled |
Hale County State Bank, Plainview, Texas 1979-10-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.