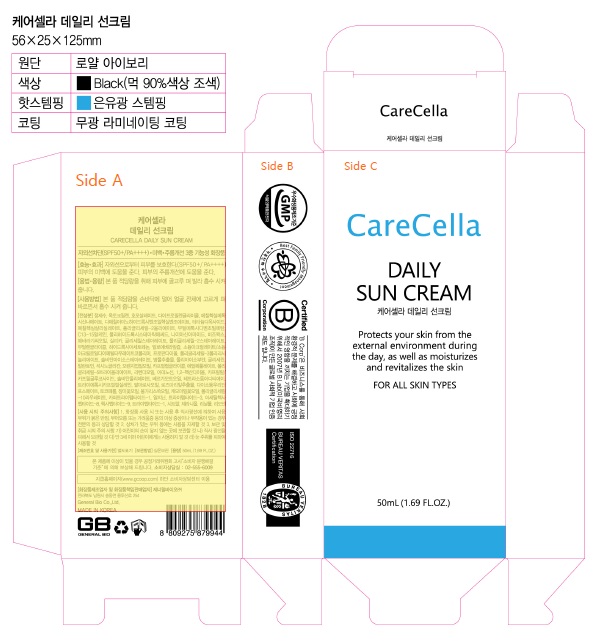
Carecella Daily Sun Cream
Carecella Daily Sun Cream by
Drug Labeling and Warnings
Carecella Daily Sun Cream by is a Otc medication manufactured, distributed, or labeled by General Bio Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CARECELLA DAILY SUN CREAM- avobenzone, homosalate, octocrylene, octinoxate, octisalate, titanium dioxide cream
General Bio Co., Ltd.
----------
Carecella Daily Sun Cream
Avobenzone (2.97%) ------------------------------------------------------------------------------- Sunscreen
Homosalate (7.5%) --------------------------------------------------------------------------------- Sunscreen
Octocrylene (7.5%) --------------------------------------------------------------------------------- Sunscreen
Octyl Methoxycinnamate (7.0%) ---------------------------------------------------------------- Sunscreen
Octyl Salicylate (5.0%) ---------------------------------------------------------------------------- Sunscreen
Titanium Dioxide (5.3%) -------------------------------------------------------------------------- Sunscreen
- Apply liberally onto the cleansed entire face and neck 15 minutes prior to sun exposure.
- Reapply as needed or after towel drying, swimming, or sweating.
- Reapply at least every 2 hours: “Sun Protection Measures." Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m.; and wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months: Ask a doctor.
- Store in a cool dry place away from direct sunlight.
- “Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun.”
1,2-Hexanediol, Caprylyl/Capryl Oligoglucoside, Acetyl Hexapeptide-8, Adenosine, Allantoin, Aloe Vera (Aloe Barbadensis) Leaf Juice, Arginine, Artemisia Vulgaris Top Oil, Bergamot Oil, Butylene Glycol, C13-15 Alkane, Caprylyl/Capryl Oligoglucoside, Caprylyl Glycol, Centella Asiatica Extract, Chamaemelum Nobile (Anthemis Nobilis) Flower Oil, Diethylamino Hydroxybenzoyl Hexyl Benzoate, Dipropylene Glycol, Edetate Sodium, Ethyl Ferulate, Glycerin, Glyceryl Monostearate, Hexapeptide-9, Hydroxyacetophenone, Lavender Oil, Niacinamide, Octinoxate, Octisalate, Orange (Citrus Aurantium Dulcis) Peel Oil Palmarosa Oil, Polyisobutylene, Polyglyceryl-10 Laurate, Polyglyceryl-2 Oleate, Polyglyceryl-2 Stearate, Polyglyceryl-3 Polyricinoleate, Polyglyceryl-5 Trioleate, Polyhydroxystearic Acid, Polyisobutylene, Prezatide, Prezatide Copper, Propanediol, Rosa Rugosa Flower Bud Oil, Rosemary (Rosmarinus Officinalis) Leaf Extract, Silicon Dioxide, Sodium Acrylate/Sodium Acryloyldimethyltaurate Copolymer, Sorbitan Isostearate, Sorbitan Monooleate, Succinoglycan, Sunflower (Helianthus Annuus) Seed Oil, Tocopherol, Triethoxycaprylylsilane, Tripeptide-3, Uridine Monophosphate Disodium, Water, Yellow Wax
| CARECELLA DAILY SUN CREAM
avobenzone, homosalate, octocrylene, octinoxate, octisalate, titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - General Bio Co., Ltd. (695273863) |
| Registrant - General Bio Co., Ltd. (695273863) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| General Bio Co., Ltd. | 695273863 | manufacture(69422-1004) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.