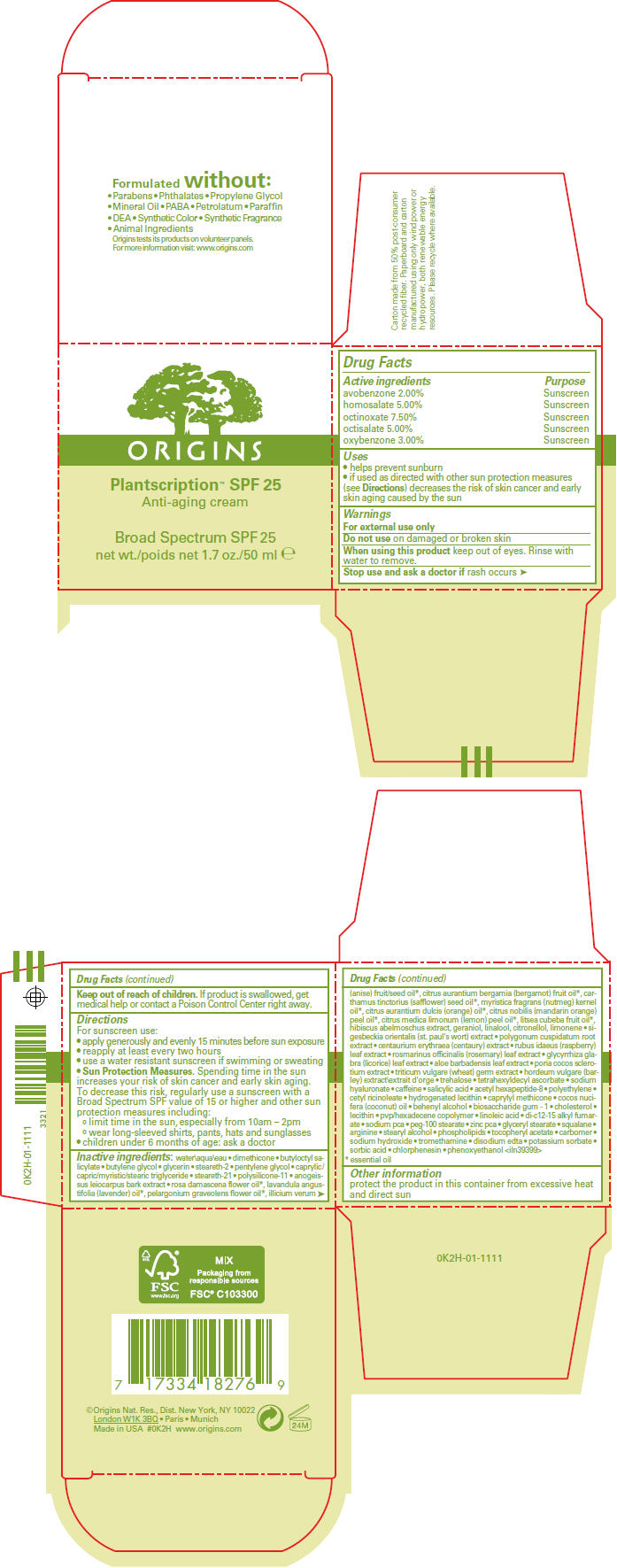
PLANTSCRIPTION ANTI-AGING BROAD SPECTRUM SPF 25- avobenzone, homosalate, octinoxate, octisalate, and oxybenzone cream
PLANTSCRIPTION ANTI-AGING BROAD SPECTRUM SPF 25 by
Drug Labeling and Warnings
PLANTSCRIPTION ANTI-AGING BROAD SPECTRUM SPF 25 by is a Otc medication manufactured, distributed, or labeled by ORIGINS NATURAL RESOURCES INC., ESTEE LAUDER COMPANY, THE, ESTEE LAUDER COSMETICS, LTD, ESTEE LAUDER COSMETICS, LTD., ESTEE LAUDER N.V., LEN-RON MANUFACTURING DIVISION OF ARAMIS INC, NORTHTEC INC, PADC 1, WHITMAN LABORATORIES, LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10am – 2pm
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau dimethicone butyloctyl salicylate butylene glycol glycerin steareth-2 pentylene glycol caprylic/capric/myristic/stearic triglyceride steareth-21 polysilicone-11 anogeissus leiocarpus bark extract rosa damascena flower oil1, lavandula angustifolia (lavender) oil1, pelargonium graveolens flower oil1, ilicium verum (anise) fruit/seed oil1, citrus aurantium bergamia (bergamot) fruit oil1, carthamus tinctorius (safflower) seed oil1, myristica fragrans (nutmeg) kernel oil1, citrus aurantium dulcis (orange) oil1, citrus nobilis (mandarin orange) peel oil1, citrus medica limonum (lemon) peel oil1, litsea cubeba fruit oil1, hibiscus abelmoschus extract, geraniol, linalool, citronellol, limonene sigesbeckia orientalis (st. paul's wort) extract polygonum cuspidatum root extract centaurium erythraea (centaury) extract rubus idaeus (raspberry) leaf extract rosmarinus officinalis (rosemary) leaf extract glycyrrhiza glabra (licorice) leaf extract aloe barbadensis leaf extract poria cocos sclerotium extract triticum vulgare (wheat) germ extract hordeum vulgare (barley) extract\extrait d'orge trehalose tetrahexyldecyl ascorbate sodium hyaluronate caffeine salicylic acid acetyl hexapeptide-8 polyethylene cetyl ricinoleate hydrogenated lecithin caprylyl methicone cocos nucifera (coconut) oil behenyl alcohol biosaccharide gum - 1 cholesterol lecithin pvp/hexadecene copolymer linoleic acid di-c12-15 alkyl fumarate sodium pca peg-100 stearate zinc pca glyceryl steaarte squalane arginine stearyl alcohol phospholipids tocopheryl acetate carbomer sodium hydroxide tromethamine disodium edta potassium sorbate sorbic acid chlorphenesin phenoxyethanol <iln39399>
- 1 essential oil
- Other information
- PRINCIPAL DISPLAY PANEL - 50 mL Jar Carton
-
INGREDIENTS AND APPEARANCE
PLANTSCRIPTION ANTI-AGING BROAD SPECTRUM SPF 25
avobenzone, homosalate, octinoxate, octisalate, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59427-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.02 g in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 0.05 g in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.075 g in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.05 g in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 0.03 g in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) dimethicone (UNII: 92RU3N3Y1O) butyloctyl salicylate (UNII: 2EH13UN8D3) butylene glycol (UNII: 3XUS85K0RA) glycerin (UNII: PDC6A3C0OX) steareth-2 (UNII: V56DFE46J5) pentylene glycol (UNII: 50C1307PZG) steareth-21 (UNII: 53J3F32P58) anogeissus leiocarpus bark (UNII: U5TP1X38RH) rosa x damascena flower oil (UNII: 18920M3T13) lavender oil (UNII: ZBP1YXW0H8) pelargonium graveolens flower oil (UNII: 3K0J1S7QGC) star anise oil (UNII: 6RXP35EIRE) bergamot oil (UNII: 39W1PKE3JI) safflower oil (UNII: 65UEH262IS) nutmeg oil (UNII: Z1CLM48948) orange oil (UNII: AKN3KSD11B) mandarin oil (UNII: NJO720F72R) lemon oil (UNII: I9GRO824LL) litsea oil (UNII: 2XIW34BN6O) geraniol (UNII: L837108USY) linalool, (+/-)- (UNII: D81QY6I88E) .beta.-citronellol, (r)- (UNII: P01OUT964K) reynoutria japonica root (UNII: 7TRV45YZF7) centaurium erythraea (UNII: 57X4TSH58S) rubus idaeus leaf (UNII: 8O2V33JG64) rosemary (UNII: IJ67X351P9) glycyrrhiza glabra leaf (UNII: GH32M797Y9) aloe vera leaf (UNII: ZY81Z83H0X) wheat germ (UNII: YR3G369F5A) trehalose (UNII: B8WCK70T7I) tetrahexyldecyl ascorbate (UNII: 9LBV3F07AZ) hyaluronate sodium (UNII: YSE9PPT4TH) caffeine (UNII: 3G6A5W338E) salicylic acid (UNII: O414PZ4LPZ) acetyl hexapeptide-8 (UNII: L4EL31FWIL) high density polyethylene (UNII: UG00KM4WR7) cetyl ricinoleate (UNII: 1P677500YD) caprylyl trisiloxane (UNII: Q95M2P1KJL) coconut oil (UNII: Q9L0O73W7L) docosanol (UNII: 9G1OE216XY) biosaccharide gum-1 (UNII: BB4PU4V09H) cholesterol (UNII: 97C5T2UQ7J) linoleic acid (UNII: 9KJL21T0QJ) di-c12-15 alkyl fumarate (UNII: A1CB3Z898P) sodium pyrrolidone carboxylate (UNII: 469OTG57A2) peg-100 stearate (UNII: YD01N1999R) zinc pidolate (UNII: C32PQ86DH4) glyceryl monostearate (UNII: 230OU9XXE4) squalane (UNII: GW89575KF9) arginine (UNII: 94ZLA3W45F) stearyl alcohol (UNII: 2KR89I4H1Y) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) sodium hydroxide (UNII: 55X04QC32I) tromethamine (UNII: 023C2WHX2V) edetate disodium (UNII: 7FLD91C86K) potassium sorbate (UNII: 1VPU26JZZ4) sorbic acid (UNII: X045WJ989B) chlorphenesin (UNII: I670DAL4SZ) phenoxyethanol (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59427-006-01 1 in 1 CARTON 11/01/2012 1 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 11/01/2012 Labeler - ORIGINS NATURAL RESOURCES INC. (611716283) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COMPANY, THE 828534516 RELABEL(59427-006) , REPACK(59427-006) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 244669714 MANUFACTURE(59427-006) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD. 202952982 MANUFACTURE(59427-006) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 MANUFACTURE(59427-006) Establishment Name Address ID/FEI Business Operations LEN-RON MANUFACTURING DIVISION OF ARAMIS INC 809771152 MANUFACTURE(59427-006) Establishment Name Address ID/FEI Business Operations NORTHTEC INC 943871157 RELABEL(59427-006) , REPACK(59427-006) Establishment Name Address ID/FEI Business Operations PADC 1 949264774 RELABEL(59427-006) , REPACK(59427-006) Establishment Name Address ID/FEI Business Operations WHITMAN LABORATORIES, LTD. 216866277 MANUFACTURE(59427-006) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 255175580 MANUFACTURE(59427-006)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.