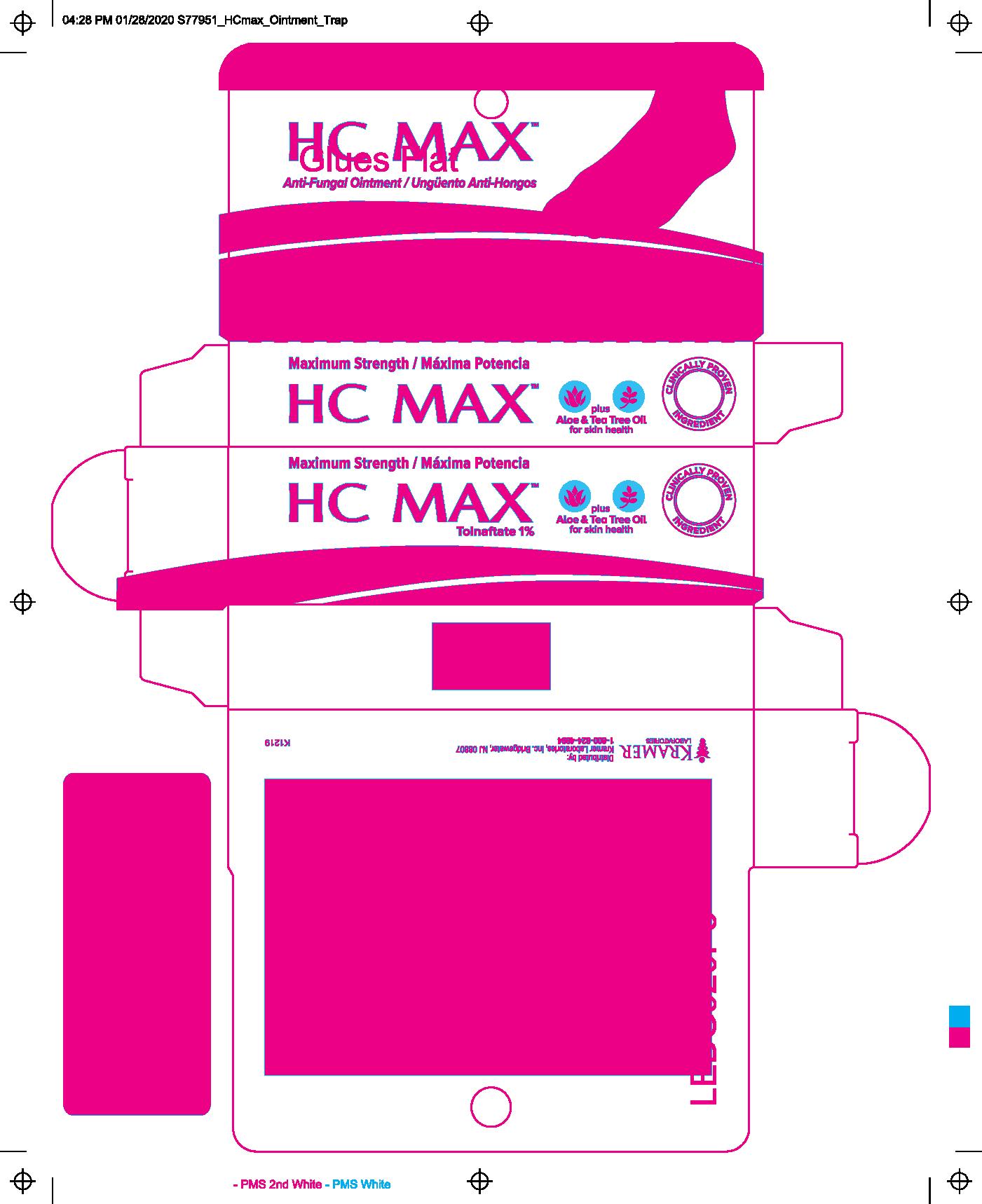
HC Max Anti-Fungal Ointment
HC Max Anti Fungal by
Drug Labeling and Warnings
HC Max Anti Fungal by is a Otc medication manufactured, distributed, or labeled by Denison Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HC MAX ANTI FUNGAL- tolnaftate 1% ointment
Denison Pharmaceuticals, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
HC Max Anti-Fungal Ointment
Uses
- Proven effective in the treatment of most athlete's foot (tinea pedis) and ringworm (tinea corporis).
- Helps prevent most athlete's foot with daily use.
- For effective relief of itching, burning and cracking.
Do not use on children under 2 years of age unless directed by a doctor.
When using this product
- avoid contact with eyes.
KEEP THIS AND ALL MEICATION OUT OF THE REACH OF CHILDREN. In case of accidental ingestion, contact a physican, emercy medical car facility or Poison Control Center immediately for advice.
Directions
- Clean affected area with soap and warm water and dry throrughy
- Apply a thin layer of HC Max TM Anti-fungal Ointment over affeted area twice daily (morning and night) or as directed by a doctor.
- Wear well-fitting, ventilated shoes, and change shoes and socks at least once dialy.
- For athlete's foot pay special atnetion to spaces between the toes.
- For athlete's foot and ringworm, use daily for 4 weeks.
- For toes fungus, apply under nail and around cuticle area. If condition persists longer, consult a doctor.
- This product is not effective on the scalp or nails.
- Supervise children in the use of this product.
Other information
Store at controlled room temperature 15º-30º C (59º-86ºF) Do not permit to freeze.
| HC MAX ANTI FUNGAL
tolnaftate 1% ointment |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Denison Pharmaceuticals, LLC (001207208) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Denison Pharmaceuticals, LLC | 001207208 | manufacture(0295-6629) | |