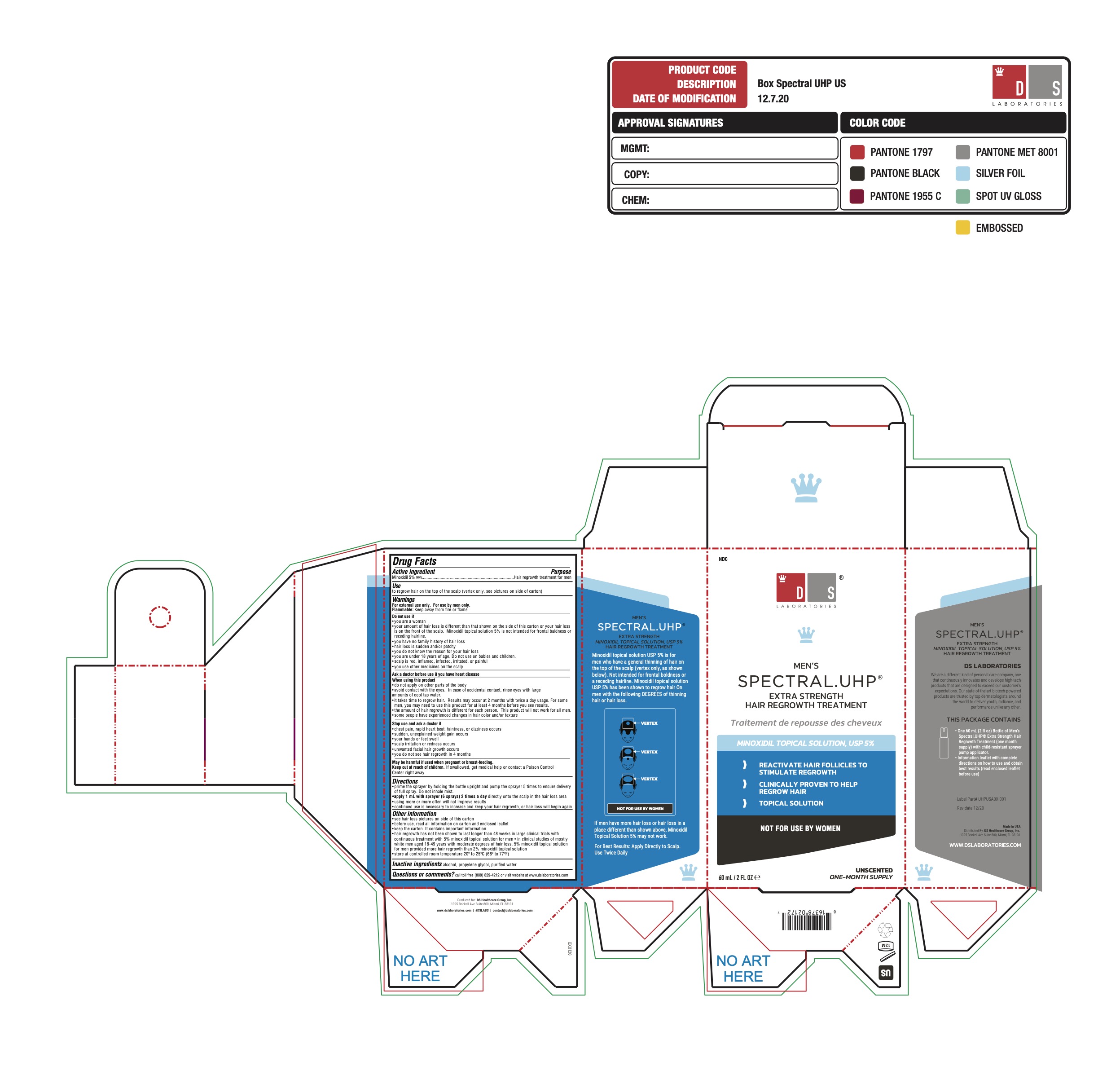
MENS SPECTRAL UHP EXTRA STRENGTH HAIR REGROWTH TREATMENT- minoxidil 5% solution
Mens SPECTRAL UHP EXTRA STRENGTH HAIR REGROWTH TREATMENT by
Drug Labeling and Warnings
Mens SPECTRAL UHP EXTRA STRENGTH HAIR REGROWTH TREATMENT by is a Otc medication manufactured, distributed, or labeled by DS Healthcare Group, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
-
DO NOT USE
Do not use if
■ You are a woman
■ Your amount of hair loss is dierent than that shown on the side of this carton, or your hair loss is on the front of the scalp.
Minoxidil topical solution 5% is not intended for frontal baldness or receding hairline.
■ You have no family history of hair loss
■ Your hair loss is sudden and/or patchy
■ You do not know the reason for your hair loss
■ You are under 18 years of age. Do not use on babies and children.
■ Your scalp is red, inflamed, infected, irritated, or painful
■ You use other medicines on the scalp
- ASK DOCTOR
-
WHEN USING
When using this product
■ Do not apply on other parts of the body
■ Avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap water.
■ It takes time to regrow hair. Results may occur at 2 months with twice a day usage. For some men, you may need to use this product
for at least 4 months before you see results.
■ The amount of hair regrowth is different for each person. This product will not work for all men.
■ Some people have experienced changes in hair color and/or texture
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- prime the sprayer by holding the bottle upright and pump the sprayer 5 times to ensure delivery
of full spray. Do not inhale mist.
- apply 1 mL with sprayer (6 sprays) 2 times a day directly onto the scalp in the hair loss area
- using more or more often will not improve results
- continued use is necessary to increase and keep your hair regrowth, or hair loss will begin again
-
OTHER INFORMATION
see hair loss pictures on side of this carton
before use, read all information on carton and enclosed leaflet
keep the carton. It contains important information.
hair regrowth has not been shown to last longer than 48 weeks in large clinical trials with
continuous treatment with 5% minoxidil topical solution for men in clinical studies of mostly
white men aged 18-49 years with moderate degrees of hair loss, 5% minoxidil topical solution
for men provided more hair regrowth than 2% minoxidil topical solution
store at controlled room temperature 20º to 25ºC (68º to 77ºF)
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MENS SPECTRAL UHP EXTRA STRENGTH HAIR REGROWTH TREATMENT
minoxidil 5% solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69188-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ISOPROPYL ALCOHOL (UNII: ND2M416302) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69188-001-01 1 in 1 BOX 12/19/2020 1 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 69188-001-02 3 in 1 BOX 12/19/2020 2 60 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076239 12/19/2020 Labeler - DS Healthcare Group, Inc. (015504134)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.