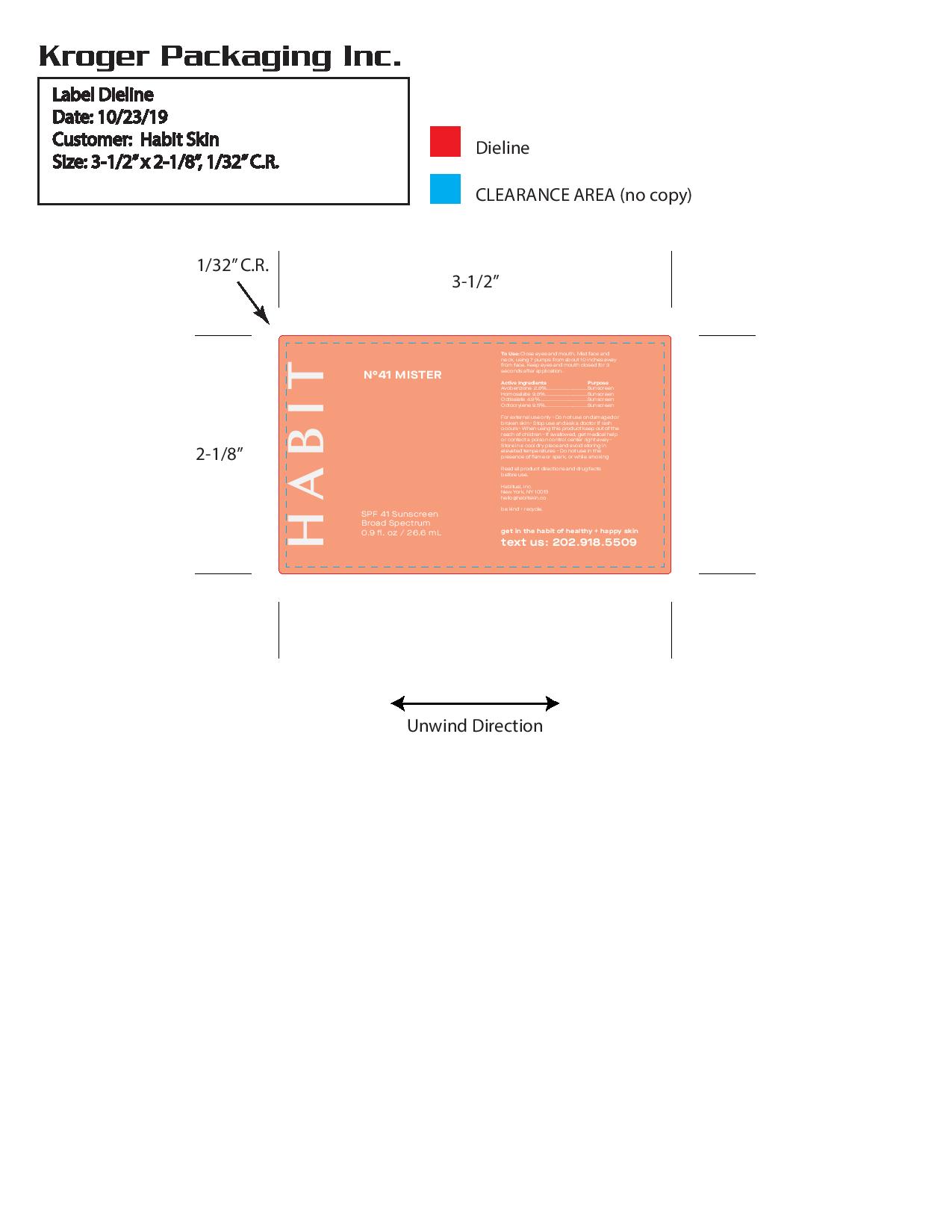
Habit SPF 41 Facial Spray
Habit by
Drug Labeling and Warnings
Habit by is a Otc medication manufactured, distributed, or labeled by Bell International Laboratories, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HABIT SPF 41- facial spray
Bell International Laboratories, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Habit SPF 41 Facial Spray
Uses
Helps prevent sunurn
If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only
Do not use on damaged or broken skin
When using this producty keep out of eyes. Rinse wtih water to remove.
Stop use and ask a doctor if rash occurs
Flammable. Keep away from fire or spark.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
Apply generously 15 minutes before sun exposure
Reapply at least every 2 hours
Use a warer-resistant sunscreen if swimming or sweating
Reapply after swimming or sweating
Children under 6 monts-age: Ask a doctor
Sun Protection Meaures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
-limit time in the sun, especially from 10a.m. - 2 p.m.
-wear long-sleeved shirts, pants, hats, and sunglasses.
Inactive Ingredients
Alcohol Denat., Bisabolol, Butyloctyl Salicylate, Capryloyl Glycerin/Sebacic Acid Copolymer, Dicaprylyl Ether, Diheptyl Succinate, Diisooctyl Succinate, Ethyl Ferulate, Isononyl Isononanoate, Lavendula Augustifolia (Lavender) Oil, Pelargonium Graveolens Flower Oil, Rosmarinus Officinalis (Rosemary) Leaf Oil
| HABIT
SPF 41
facial spray |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Bell International Laboratories, Inc (967781555) |
Trademark Results [Habit]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HABIT 98432226 not registered Live/Pending |
The Habit Restaurants, LLC 2024-03-04 |
 HABIT 98238352 not registered Live/Pending |
ZURU EDGE LIMITED 2023-10-24 |
 HABIT 98150647 not registered Live/Pending |
Sugar and Hive LLC 2023-08-25 |
 HABIT 98088002 not registered Live/Pending |
Habitual, Inc. 2023-07-17 |
 HABIT 98053289 not registered Live/Pending |
Sugar and Hive LLC 2023-06-21 |
 HABIT 97343236 not registered Live/Pending |
3550 Cadillac Ave LLC 2022-04-01 |
 HABIT 97325295 not registered Live/Pending |
Gallo, AJ 2022-03-22 |
 HABIT 97054896 not registered Live/Pending |
Simple Design Ltd. 2021-09-30 |
 HABIT 90799097 not registered Live/Pending |
Cycling Sports Group, Inc. 2021-06-28 |
 HABIT 90774327 not registered Live/Pending |
Mahco, Inc. 2021-06-15 |
 HABIT 90774281 not registered Live/Pending |
Mahco, Inc. 2021-06-15 |
 HABIT 90774231 not registered Live/Pending |
Mahco, Inc. 2021-06-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.