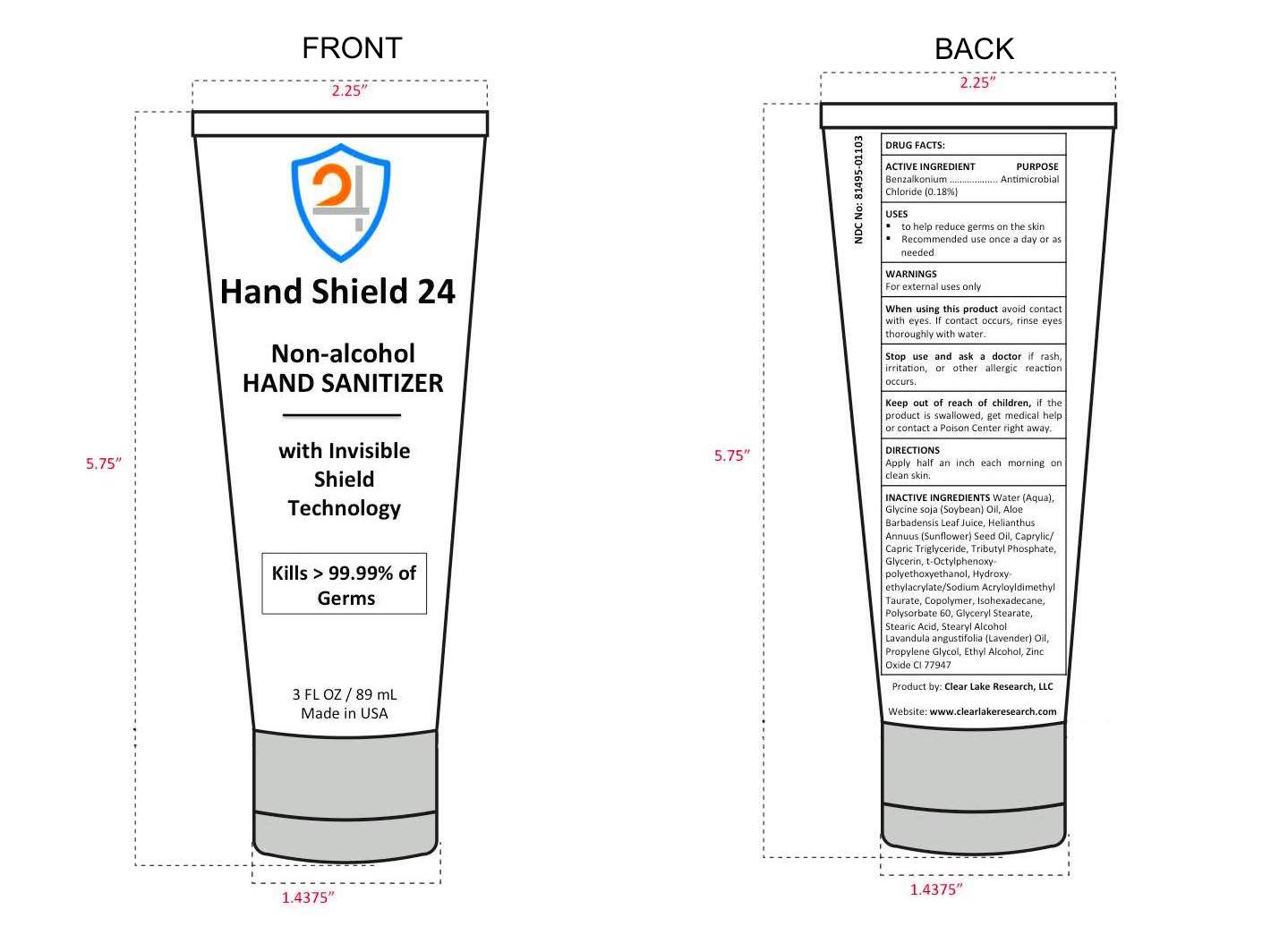
HAND SHIELD 24 HAND SANITIZER by CLEAR LAKE RESEARCH, LLC / Clear Lake Research, LLC
HAND SHIELD 24 HAND SANITIZER by
Drug Labeling and Warnings
HAND SHIELD 24 HAND SANITIZER by is a Otc medication manufactured, distributed, or labeled by CLEAR LAKE RESEARCH, LLC, Clear Lake Research, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HAND SHIELD 24 HAND SANITIZER- benzalkonium chloride cream
CLEAR LAKE RESEARCH, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
When using this product
When using this product avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor
Stop use and ask a doctor if rash, irritation, or other allergic reaction occurs
Keep Out of Reach of Children
Keep out of reach of children, if the product is swallowed, get medical help or contact a Poison Center right away.
Inactive Ingredients
Water (Aqua), Glycine soja (Soybean) Oil, Aloe Barbadensis Leaf Juice, Helianthus Annuus (Sunflower) Seed Oil, Caprylic/Capric Triglyceride, Tributyl Phosphate, Glycerin, t-Octylphenoxy-polyethoxyethanol, Hydroxy-ethylacrylate/Sodium Acryloyldimethyl Taurate, Copolymer, Isohexadecane, Polysorbate 60, Glyceryl Stearate, Stearic Acid, Stearyl Alcohol, Lavandula angustifolia (Lavender) Oil, Propylene Glycol, Ethyl Alcohol, Zinc Oxide CI 77947
| HAND SHIELD 24 HAND SANITIZER
benzalkonium chloride cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - CLEAR LAKE RESEARCH, LLC (077493647) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Clear Lake Research, LLC | 077493647 | label(81495-111) | |