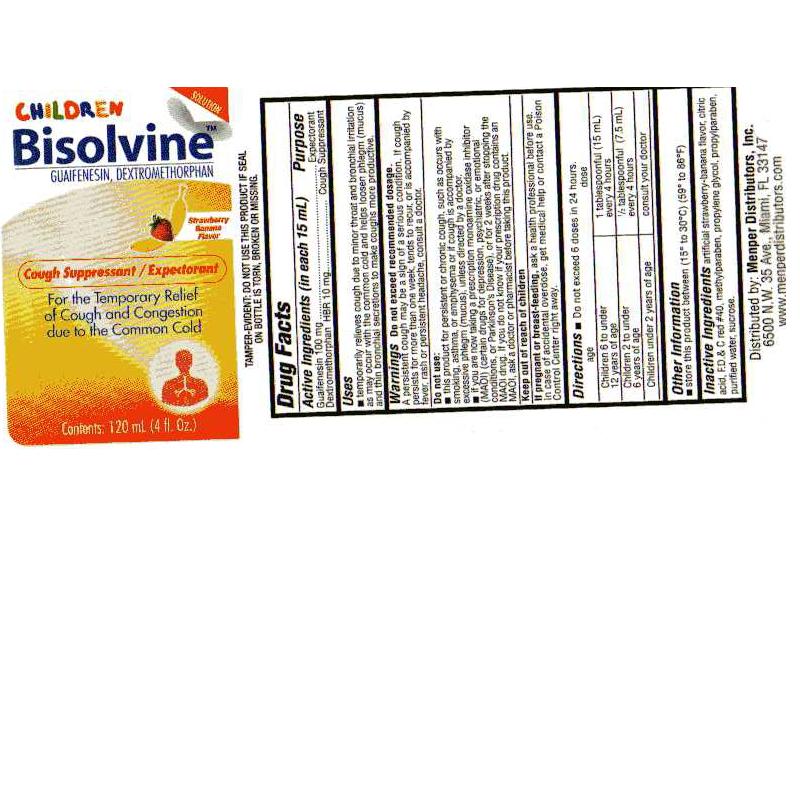
BISOLVINE CHILD- guaifenesin, dextromethorphan liquid
Bisolvine by
Drug Labeling and Warnings
Bisolvine by is a Otc medication manufactured, distributed, or labeled by Pharmalab Enterprises Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
-
DO NOT USE
Do not use:
* this product for persistent or chronic cough, such as occurs with smoking, asthma, or emphysema or if cough is accompanied by excessive phlegm (mucus), unless directed by a doctor.
* If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's Disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- Other Information
- INACTIVE INGREDIENT
- Directions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BISOLVINE CHILD
guaifenesin, dextromethorphan liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 14505-488 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 100 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 10 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C RED NO. 40 (UNII: WZB9127XOA) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SUCROSE (UNII: C151H8M554) Product Characteristics Color Score Shape Size Flavor STRAWBERRY (Strawberry -Banana Flavor) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 14505-488-04 120 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 02/18/2010 Labeler - Pharmalab Enterprises Inc. (174401088) Registrant - Pharmalab Enterprises Inc. (174401088) Establishment Name Address ID/FEI Business Operations Pharmalab Enterprises Inc. 174401088 manufacture
Trademark Results [Bisolvine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BISOLVINE 77826772 3780920 Live/Registered |
ALPER HOLDINGS INC. 2009-09-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.