Once DailyMethylphenidate Hydrochloride Extended-release Capsules CII
Methylphenidate Hydrochloride by
Drug Labeling and Warnings
Methylphenidate Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by CorePharma, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
METHYLPHENIDATE HYDROCHLORIDE- methylphenidate hydrochloride capsule, extended release
CorePharma, LLC
----------
Once Daily
Methylphenidate Hydrochloride Extended-release Capsules CII
Rx only
DESCRIPTION
Methylphenidate hydrochloride extended-release capsules are a central nervous system (CNS) stimulant. The extended-release capsules comprise beads each with both immediate-release (IR) and extended-release (ER) components such that 30% of the dose is provided by the IR component and 70% of the dose is provided by the ER component. Methylphenidate hydrochloride extended-release capsules are available in six strengths containing 10 mg (3 mg IR; 7 mg ER), 20 mg (6 mg IR; 14 mg ER), 30 mg (9 mg IR; 21 mg ER), 40 mg (12 mg IR; 28 mg ER), 50 mg (15 mg IR; 35 mg ER), or 60 mg (18 mg IR; 42 mg ER) of methylphenidate hydrochloride for oral administration.
Chemically, methylphenidate hyrochloride is d,l (racemic)-threo-methyl α-phenyl-2-piperidineacetate hydrochloride. Its empirical formula is C14H19NO2HCl. Its structural formula is:
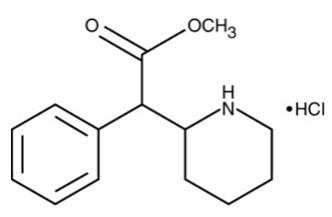
Methylphenidate hydrochloride, USP is a white, odorless, crystalline powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. Its molecular weight is 269.77.
Methylphenidate hydrochloride extended-release capsules also contain the following inert ingredients: Sugar spheres, povidone, hydroxypropylmethylcellulose and polyethylene glycol, ethyl cellulose, cetyl alcohol, sodium lauryl sulfate, dibutyl sebacate, gelatin, and titanium dioxide.
The individual capsules contain the following color agents:
10 mg capsules: FD&C Blue No. 2, FDA/E172 Yellow Iron Oxide
20 mg capsules: D&C Red No. 28, FD&C Blue No. 1, FD&C Green No. 3
30 mg capsules: FDA/E172 Black Iron Oxide, FDA/E172 Red Iron Oxide, FDA/E172 Yellow Iron Oxide
40 mg capsules: D&C Yellow No. 10, FD&C Red No. 40
50 mg capsules: D&C Red No. 28, FD&C Green No. 3, FDA/E172 Black Iron Oxide
CLINICAL PHARMACOLOGY
Pharmacodynamics
Methylphenidate HCl is a central nervous system (CNS) stimulant. The mode of therapeutic action in Attention Deficit Hyperactivity Disorder (ADHD) is not known. Methylphenidate is thought to block the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space. Methylphenidate is a racemic mixture comprised of the d- and l-threo enantiomers. The d-threo enantiomer is more pharmacologically active than the l-threo enantiomer.
Pharmacokinetics
The pharmacokinetics of the methylphenidate hydrochloride extended-release capsule methylphenidate hydrochloride formulation have been studied in healthy adult volunteers and in children with Attention Deficit Hyperactivity Disorder (ADHD).
Absorption And Distribution
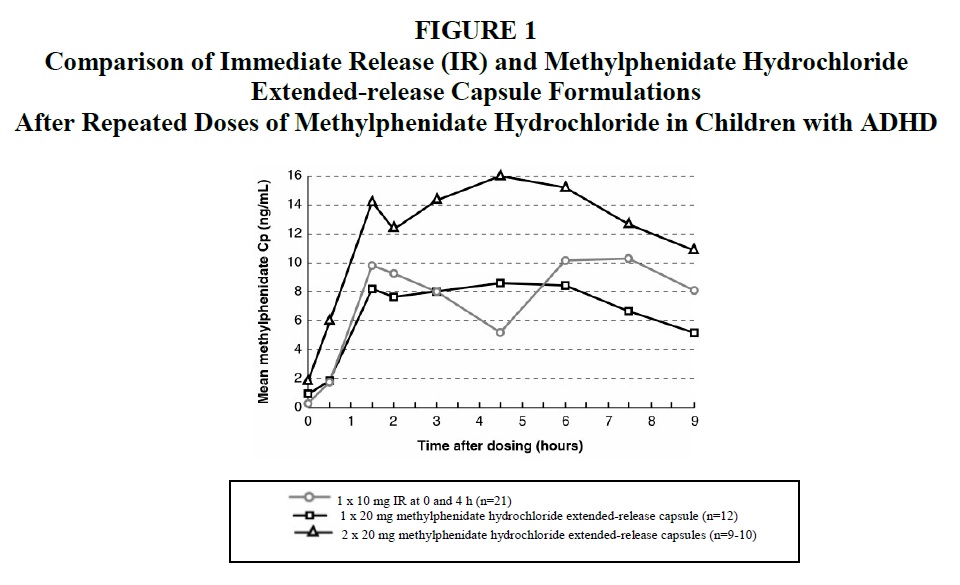
Methylphenidate is readily absorbed. Methylphenidate hydrochloride extended-release capsules have a plasma/time concentration profile showing two phases of drug release with a sharp, initial slope similar to a methylphenidate immediate-release tablet, and a second rising portion approximately three hours later, followed by a gradual decline. (See Figure 1 below.)
Comparison Of Immediate Release (IR) And Methylphenidate Hydrochloride Extended-release Capsules Formulations After Repeated Doses Of Methylphenidate HCl In Children With ADHD
Methylphenidate hydrochloride extended-release capsules were administered as repeated once-daily doses of 20 mg or 40 mg to children aged 7-12 years with ADHD for one week. After a dose of 20 mg, the mean (±SD) early Cmax was 8.6 (±2.2) ng/mL, the later Cmax was 10.9 (±3.9)* ng/mL and AUC0-9h was 63.0 (±16.8) ngh/mL. The corresponding values after a 40 mg dose were 16.8 (±5.1) ng/mL, 15.1 (±5.8)* ng/mL and 120 (±39.6) ngh/mL, respectively. The early peak concentrations (median) were reached about 1.5 hours after dose intake, and the second peak concentrations (median) were reached about 4.5 hours after dose intake. The means for Cmax and AUC following a dose of 20 mg were slightly lower than those seen with 10 mg of the immediate-release formulation, dosed at 0 and 4 hours.
*25-30% of the subjects had only one observed peak (Cmax) concentration of methylphenidate.
Dose Proportionality
Following single oral doses of 10-60 mg methylphenidate free base as a solution given to ten healthy male volunteers, Cmax and AUC increased proportionally with increasing doses. After the 60 mg dose, tmax was reached 1.5 hours post-dose, with a mean Cmax of 31.8 ng/mL (range 24.7-40.9 ng/mL).
Following one week of repeated once-daily doses of 20 mg or 40 mg methylphenidate hydrochloride extended-release capsules to children aged 7-12 years with ADHD, Cmax and AUC were proportional to the administered dose.
Food Effects
In a study in adult volunteers to investigate the effects of a high-fat meal on the bioavailability of a dose of 40 mg, the presence of food delayed the early peak by approximately 1 hour (range -2 to 5 hours delay). The plasma levels rose rapidly following the food-induced delay in absorption. Overall, a high-fat meal increased the Cmax of methylphenidate hydrochloride extended-release capsules by about 30% and AUC by about 17%, on average (see DOSAGE and ADMINISTRATION).
After a single dose, the bioavailability (Cmax and AUC) of methylphenidate in 26 healthy adults was unaffected by sprinkling the capsule contents on applesauce as compared to the intact capsule. This finding demonstrates that a 20 mg methylphenidate hydrochloride extended-release capsule, when opened and sprinkled on one tablespoon of applesauce, is bioequivalent to the intact capsule.
Metabolism And Excretion
In humans, methylphenidate is metabolized primarily via deesterification to alpha-phenyl-piperidine acetic acid (ritalinic acid). The metabolite has little or no pharmacologic activity.
In vitro studies showed that methylphenidate was not metabolized by cytochrome P450 isoenzymes, and did not inhibit cytochrome P450 isoenzymes at clinically observed plasma drug concentrations.
The mean terminal half-life (t½) of methylphenidate following administration of methylphenidate hydrochloride extended-release capsules (t½=6.8h) is longer than the mean terminal (t½) following administration of methylphenidate hydrochloride immediate-release tablets (t½=2.9h) and methylphenidate hydrochloride sustained-release tablets (t½=3.4h) in healthy adult volunteers. This suggests that the elimination process observed for methylphenidate hydrochloride extended-release capsules is controlled by the release rate of methylphenidate from the extended-release formulation, and that the drug absorption is the rate-limiting process.
Alcohol Effect
An in vitro study was conducted to explore the effect of alcohol on the release characteristics of methylphenidate from the methylphenidate hydrochloride extended-release 60 mg capsule dosage form. At an alcohol concentration of 40% there was an increase in the release rate of methylphenidate in the first hour, resulting in 84% of the methylphenidate being released. The results with the 60 mg capsule are considered to be representative of the other available capsule strengths. Patients should be advised to avoid alcohol while taking methylphenidate hydrochloride extended-release capsules.
Special Populations
Gender
The pharmacokinetics of methylphenidate after a single dose of methylphenidate hydrochloride extended-release capsules were similar between adult men and women.
Race
The influence of race on the pharmacokinetics of methylphenidate after methylphenidate hydrochloride extended-release capsules administration has not been studied.
Age
The pharmacokinetics of methylphenidate after methylphenidate hydrochloride extended-release capsules administration have not been studied in children less than 6 years of age.
Renal Insufficiency
There is no experience with the use of methylphenidate hydrochloride extended-release capsules in patients with renal insufficiency. After oral administration of radiolabeled methylphenidate in humans, methylphenidate was extensively metabolized and approximately 80% of the radioactivity was excreted in the urine in the form of ritalinic acid. Since renal clearance is not an important route of methylphenidate clearance, renal insufficiency is expected to have little effect on the pharmacokinetics of methylphenidate hydrochloride extended-release capsules.
Hepatic Insufficiency
There is no experience with the use of methylphenidate hydrochloride extended-release capsules in patients with hepatic insufficiency.
CLINICAL STUDIES
Methylphenidate hydrochloride extended-release capsules were evaluated in a double-blind, parallel-group, placebo-controlled trial in which 321 untreated or previously treated pediatric patients with a DSM-IV diagnosis of Attention Deficit Hyperactivity Disorder (ADHD), 6 to 15 years of age, received a single morning dose for up to 3 weeks. Patients were required to have the combined or predominantly hyperactive-impulsive subtype of ADHD; patients with the predominantly inattentive subtype were excluded. Patients randomized to the methylphenidate hydrochloride extended-release capsules group received 20 mg daily for the first week. Their dosage could be increased weekly to a maximum of 60 mg by the third week, depending on individual response to treatment.
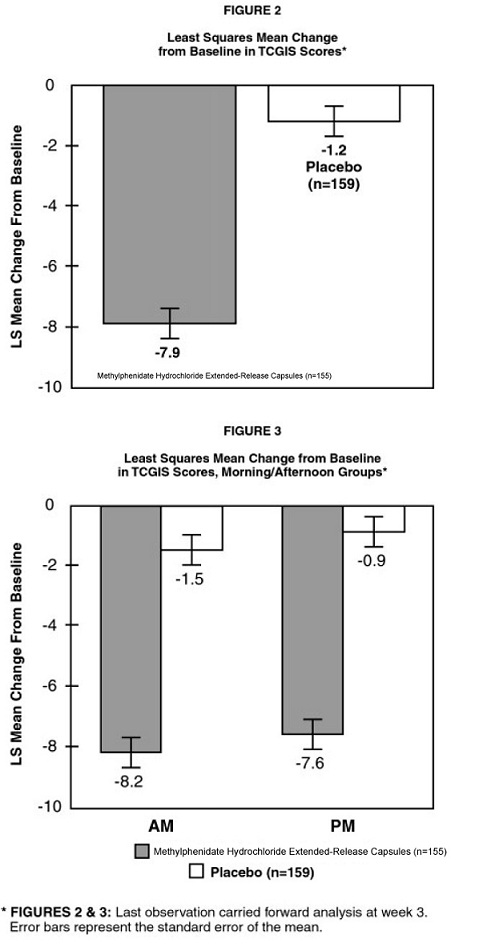
The patient’s regular school teacher completed the teachers’ version of the Conners’ Global Index Scale (TCGIS), a scale for assessing ADHD symptoms, in the morning and again in the afternoon on three alternate days of each treatment week. The change from baseline of the overall average (i.e., an average of morning and afternoon scores over 3 days) of the total TCGIS scores during the last week of treatment was analyzed as the primary efficacy parameter. Patients treated with methylphenidate hydrochloride extended-release capsules showed a statistically significant improvement in symptom scores from baseline over patients who received placebo. (See Figure 2.) Separate analyses of TCGIS scores in the morning and afternoon revealed superiority in improvement with methylphenidate hydrochloride extended-release capsules over placebo during both time periods. (See Figure 3.) This demonstrates that a single morning dose of methylphenidate hydrochloride extended-release capsules exerts a treatment effect in both the morning and the afternoon.
INDICATION AND USAGE
Attention Deficit Hyperactivity Disorder (ADHD)
Methylphenidate hydrochloride extended-release capsules are indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD).
The efficacy of methylphenidate hydrochloride extended-release capsules in the treatment of ADHD was established in one controlled trial of children aged 6 to 15 who met DSM-IV criteria for ADHD (see CLINICAL PHARMACOLOGY).
A diagnosis of Attention Deficit Hyperactivity Disorder (ADHD; DSM-IV) implies the presence of hyperactive-impulsive or inattentive symptoms that caused impairment and were present before age 7 years. The symptoms must cause clinically significant impairment, e.g., in social, academic, or occupational functioning, and be present in two or more settings, e.g., school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. For the Inattentive Type, at least six of the following symptoms must have persisted for at least 6 months: lack of attention to details/careless mistakes; lack of sustained attention; poor listener; failure to follow through on tasks; poor organization; avoids tasks requiring sustained mental effort; loses things; easily distracted; forgetful. For the Hyperactive-Impulsive Type, at least six of the following symptoms must have persisted for at least 6 months: fidgeting/squirming; leaving seat; inappropriate running/climbing; difficulty with quiet activities; “on the go;” excessive talking; blurting answers; can’t wait turn; intrusive. The Combined Types requires both inattentive and hyperactive-impulsive criteria to be met.
Special Diagnostic Considerations
Specific etiology of this syndrome is unknown, and there is no single diagnostic test. Adequate diagnosis requires the use not only of medical but of special psychological, educational, and social resources. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the child and not solely on the presence of the required number of DSM-IV characteristics.
Need For Comprehensive Treatment Program
Methylphenidate hydrochloride extended-release capsules are indicated as an integral part of a total treatment program for ADHD that may include other measures (psychological, educational, social) for patients with this syndrome. Drug treatment may not be indicated for all children with this syndrome. Stimulants are not intended for use in the child who exhibits symptoms secondary to environmental factors and/or other primary psychiatric disorders, including psychosis. Appropriate educational placement is essential and psychosocial intervention is often helpful. When remedial measures alone are insufficient, the decision to prescribe stimulant medication will depend upon the physician’s assessment of the chronicity and severity of the child’s symptoms.
Long-Term Use
The effectiveness of methylphenidate hydrochloride extended-release capsules for long-term use, i.e., for more than 3 weeks, has not been systematically evaluated in controlled trials. Therefore, the physician who elects to use methylphenidate hydrochloride extended-release capsules for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient (see DOSAGE and ADMINISTRATION).
CONTRAINDICATIONS
Agitation
Methylphenidate hydrochloride extended-release capsules are contraindicated in patients with marked anxiety, tension and agitation, since the drug may aggravate these symptoms.
Hypersensitivity To Methylphenidate Or Other Excipients
Methylphenidate hydrochloride extended-release capsules are contraindicated in patients known to be hypersensitive to methylphenidate or other components of the product.
Methylphenidate hydrochloride extended-release capsules contain sucrose. Therefore, patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption, or sucrase-isomaltase insufficiency should not take this medicine.
Glaucoma
Methylphenidate hydrochloride extended-release capsules are contraindicated in patients with glaucoma.
Tics
Methylphenidate hydrochloride extended-release capsules are contraindicated in patients with motor tics or with a family history or diagnosis of Tourette’s syndrome (see ADVERSE REACTIONS).
Monoamine Oxidase Inhibitors
Methylphenidate hydrochloride extended-release capsules are contraindicated during treatment with monoamine oxidase inhibitors, and also within a minimum of 14 days following discontinuation of a monoamine oxidase inhibitor (hypertensive crises may result).
Hypertension And Other Cardiovascular Conditions
Methylphenidate hydrochloride extended-release capsules are contraindicated in patients with severe hypertension, angina pectoris, cardiac arrhythmias, heart failure, recent myocardial infarction, hyperthyroidism or thyrotoxicosis (see WARNINGS).
WARNINGS
Serious Cardiovascular Events
Sudden Death And Pre-existing Structural Cardiac Abnormalities Or Other Serious Heart Problems
Children And Adolescents
Sudden death has been reported in association with CNS stimulant treatment at usual doses in children and adolescents with structural cardiac abnormalities or other serious heart problems. Although some serious heart problems alone carry an increased risk of sudden death, stimulant products generally should not be used in children or adolescents with known serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug (see CONTRAINDICATIONS).
Adults
Sudden deaths, stroke, and myocardial infarction have been reported in adults taking stimulant drugs at usual doses for ADHD. Although the role of stimulants in these adult cases is also unknown, adults have a greater likelihood than children of having serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other serious cardiac problems. Adults with such abnormalities should also generally not be treated with stimulant drugs (see CONTRAINDICATIONS).
Hypertension And Other Cardiovascular Conditions
Stimulant medications cause a modest increase in average blood pressure (about 2-4 mmHg) and average heart rate (about 3-6 bpm), and individuals may have larger increases. While the mean changes alone would not be expected to have short-term consequences, all patients should be monitored for larger changes in heart rate and blood pressure. Caution is indicated in treating patients whose underlying medical conditions might be compromised by increases in blood pressure or heart rate, e.g., those with pre-existing hypertension, heart failure, recent myocardial infarction, or ventricular arrhythmia (see CONTRAINDICATIONS).
Assessing Cardiovascular Status In Patients Being Treated With Stimulant Medications
Children, adolescents, or adults who are being considered for treatment with stimulant medications should have a careful history (including assessment for a family history of sudden death or ventricular arrhythmia) and physical exam to assess for the presence of cardiac disease, and should receive further cardiac evaluation if findings suggest such disease (e.g., electrocardiogram and echocardiogram). Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during stimulant treatment should undergo a prompt cardiac evaluation.
Psychiatric Adverse Events
Pre-Existing Psychosis
Administration of stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.
Bipolar Illness
Particular care should be taken in using stimulants to treat ADHD in patients with comorbid bipolar disorder because of concern for possible induction of a mixed/manic episode in such patients. Prior to initiating treatment with a stimulant, patients with comorbid depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.
Emergence Of New Psychotic Or Manic Symptoms
Treatment emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without prior history of psychotic illness or mania can be caused by stimulants at usual doses. If such symptoms occur, consideration should be given to a possible causal role of the stimulant, and discontinuation of treatment may be appropriate. In a pooled analysis of multiple short-term, placebo-controlled studies, such symptoms occurred in about 0.1% (4 patients with events out of 3482 exposed to methylphenidate or amphetamine for several weeks at usual doses) of stimulant-treated patients compared to 0 in placebo-treated patients.
Aggression
Aggressive behavior or hostility is often observed in children and adolescents with ADHD, and has been reported in clinical trials and the postmarketing experience of some medications indicated for the treatment of ADHD. Although there is no systematic evidence that stimulants cause aggressive behavior or hostility, patients beginning treatment for ADHD should be monitored for the appearance of or worsening of aggressive behavior or hostility.
Long-Term Suppression Of Growth
Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated children over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated children (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development. Published data are inadequate to determine whether chronic use of amphetamines may cause a similar suppression of growth, however, it is anticipated that they likely have this effect as well. Therefore, growth should be monitored during treatment with stimulants, and patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
Seizures
There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in absence of seizures, and, very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, the drug should be discontinued.
Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate products in both pediatric and adult patients. Priapism was not reported with drug initiation but developed after some time on the drug, often subsequent to an increase in dose. Priapism has also appeared during a period of drug withdrawal (drug holidays or discontinuation). Patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
Peripheral Vasculopathy, including Raynaud's phenomenon
Stimulants, including methylphenidate hydrochloride extended-release capsules, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud’s phenomenon, were observed in post-marketing reports at different times and at therapeutic doses in all age groups throughout the course of treatment. Signs and symptoms generally improve after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Visual Disturbance
Difficulties with accommodation and blurring of vision have been reported with stimulant treatment.
Use In Children Under Six Years Of Age
Methylphenidate hydrochloride extended-release capsules should not be used in children under six years, since safety and efficacy in this age group have not been established.
|
Drug Dependence |
PRECAUTIONS
Hematologic Monitoring
Periodic CBC, differential, and platelet counts are advised during prolonged therapy.
Drug Testing
Methylphenidate hydrochloride extended-release capsules contain methylphenidate which may result in a positive result during drug testing.
Information For Patients
Patients should be instructed to take one dose in the morning before breakfast. The patients should be instructed that the capsule may be swallowed whole, or alternatively, the capsule may be opened and the capsule contents sprinkled onto a small amount (tablespoon) of applesauce and given immediately, and not stored for future use. The capsules and the capsule contents must not be crushed or chewed.
Patients should be advised to avoid alcohol while taking methylphenidate hydrochloride extended-release capsules. Consumption of alcohol while taking methylphenidate hydrochloride extended-release capsules may result in a more rapid release of the dose of methylphenidate.
Priapism
Advise patients, caregivers, and family members of the possibility of painful or prolonged penile erections (priapism). Instruct the patient to seek immediate medical attention in the event of priapism.
Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud’s phenomenon]
- Instruct patients beginning treatment with methylphenidate hydrochloride extended-release capsules about the risk of peripheral vasculopathy, including Raynaud’s Phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking methylphenidate hydrochloride extended-release capsules.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with methylphenidate and should counsel them in its appropriate use. A patient Medication Guide is available for methylphenidate hydrochloride extended-release capsules. The prescriber or healthcare professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document. The Medication Guide may also be found in the full prescribing information for methylphenidate hydrochloride extended-release capsules on www.corepharma.com or by calling 1-800-850-2719.
Drug Interactions
Because of possible effects on blood pressure, methylphenidate hydrochloride extended-release capsules should be used cautiously with pressor agents.
Human pharmacologic studies have shown that methylphenidate may inhibit the metabolism of coumarin anticoagulants, anticonvulsants (e.g., phenobarbital, phenytoin, primidone), phenylbutazone and some antidepressants (tricyclics and selective serotonin reuptake inhibitors). Downward dose adjustment of these drugs may be required when given concomitantly with methylphenidate. It may be necessary to adjust the dosage and monitor plasma drug concentrations (or, in the case of coumarin, coagulation times), when initiating or discontinuing concomitant methylphenidate.
In theory, there is a possibility that the clearance of methylphenidate might be affected by urinary pH, either being increased with acidifying agents or decreased with alkalizing agents. This should be considered when methylphenidate is given in combination with agents that alter urinary pH.
Halogenated Anesthetics
There is a risk of sudden blood pressure increase during surgery. If surgery is planned, methylphenidate hydrochloride extended-release capsules should not be taken the day of the surgery.
Carcinogenesis, Mutagenesis, And Impairment Of Fertility
In a lifetime carcinogenicity study carried out in B6C3F1 mice, methylphenidate caused an increase in hepatocellular adenomas and, in males only, an increase in hepatoblastomas, at a daily dose of approximately 60 mg/kg/day. This dose is approximately 30 times and 4 times the maximum recommended human dose of methylphenidate hydrochloride extended-release capsules on a mg/kg and mg/m2 basis, respectively. Hepatoblastoma is a relatively rare rodent malignant tumor type. There was no increase in total malignant hepatic tumors. The mouse strain used is sensitive to the development of hepatic tumors, and the significance of these results to humans is unknown.
Methylphenidate did not cause any increases in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day, which is approximately 22 times and 5 times the maximum recommended human dose of methylphenidate hydrochloride extended-release capsules on a mg/kg and mg/m2 basis, respectively.
In a 24-week carcinogenicity study in the transgenic mouse strain p53+/-, which is sensitive to genotoxic carcinogens, there was no evidence of carcinogenicity. Male and female mice were fed diets containing the same concentration of methylphenidate as in the lifetime carcinogenicity study; the high-dose groups were exposed to 60 to 74 mg/kg/day of methylphenidate.
Methylphenidate was not mutagenic in the in vitro Ames reverse mutation assay or in the in vitro mouse lymphoma cell forward mutation assay. Sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response, in an in vitro assay in cultured Chinese Hamster Ovary cells. Methylphenidate was negative in vivo in males and females in the mouse bone marrow micronucleus assay.
Methylphenidate did not impair fertility in male or female mice that were fed diets containing the drug in an 18-week Continuous Breeding study. The study was conducted at doses up to 160 mg/kg/day, approximately 80-fold and 8-fold the highest recommended human dose of methylphenidate hydrochloride extended-release capsules on a mg/kg and mg/m2 basis, respectively.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Methylphenidate has been shown to have teratogenic effects in rabbits when given in doses of 200 mg/kg/day, which is approximately 100 times and 40 times the maximum recommended human dose on a mg/kg and mg/m2 basis, respectively.
A reproduction study in rats revealed no evidence of teratogenicity at an oral dose of 58 mg/kg/day. However, this dose, which caused some maternal toxicity, resulted in decreased postnatal pup weights and survival when given to the dams from day one of gestation through the lactation period. This dose is approximately 30 fold and 6 fold the maximum recommended human dose of methylphenidate hydrochloride extended-release capsules on a mg/kg and mg/m2 basis, respectively.
There are no adequate and well-controlled studies in pregnant women. Methylphenidate hydrochloride extended-release capsules should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether methylphenidate is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised if methylphenidate hydrochloride extended-release capsules are administered to a nursing woman.
Pediatric Use
The safety and efficacy of methylphenidate hydrochloride extended-release capsules in children under 6 years old have not been established. Long-term effects of methylphenidate in children have not been well established (see WARNINGS).
ADVERSE REACTIONS
The premarketing development program for methylphenidate hydrochloride extended-release capsules included exposures in a total of 228 participants in clinical trials (188 pediatric patients with ADHD, 40 healthy adult subjects). These participants received methylphenidate hydrochloride extended-release capsules 20, 40, and/or 60 mg/day. The 188 patients (ages 6 to 15) were evaluated in one controlled clinical study, one controlled, crossover clinical study, and one uncontrolled clinical study. Safety data on all patients are included in the discussion that follows. Adverse reactions were assessed by collecting adverse events, results of physical examinations, vital signs, weights, laboratory analyses, and ECGs.
Adverse events during exposure were obtained primarily by general inquiry and recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of events into a smaller number of standardized event categories. In the tables and listings that follow, COSTART terminology has been used to classify reported adverse events.
The stated frequencies of adverse events represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse event of the type listed. An event was considered treatment emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
Adverse Findings In Clinical Trials With Methylphenidate Hydrochloride Extended-release Capsules
Adverse Events Associated With Discontinuation Of Treatment
In the 3-week placebo-controlled, parallel-group trial, two methylphenidate hydrochloride extended-release capsules-treated patients (1%) and no placebo-treated patients discontinued due to an adverse event (rash and pruritus; and headache, abdominal pain, and dizziness, respectively).
Adverse Events Occurring At An Incidence Of 5% Or More Among Methylphenidate Hydrochloride Extended-release Capsules-Treated Patients
Table 1 enumerates, for a pool of the three studies in pediatric patients with ADHD, at methylphenidate hydrochloride extended-release capsules doses of 20, 40, or 60 mg/day, the incidence of treatment-emergent adverse events. One study was a 3-week placebo-controlled, parallel-group trial, one study was a controlled, crossover trial, and the third study was an open titration trial. The table includes only those events that occurred in 5% or more of patients treated with methylphenidate hydrochloride extended-release capsules where the incidence in patients treated with methylphenidate hydrochloride extended-release capsules was greater than the incidence in placebo-treated patients.
The prescriber should be aware that these figures cannot be used to predict the incidence of adverse events in the course of usual medical practice where patient characteristics and other factors differ from those which prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and non-drug factors to the adverse event incidence rate in the population studied.
| Table 1 Incidence of Treatment-Emergent Events1 in a Pool of 3-4 Week Clinical Trials of Methylphenidate Hydrochloride Extended-release Capsules |
|||
|
Body System | Preferred Term | Methylphenidate Hydrochloride Extended-release Capsules
(n=188) | Placebo
(n=190) |
| General | Headache Abdominal pain (stomach ache) | 12% 7% | 8% 4% |
| Digestive System | Anorexia (loss of appetite) | 9% | 2% |
| Nervous System | Insomnia | 5% | 2% |
1: Events, regardless of causality, for which the incidence for patients treated with methylphenidate hydrochloride extended-release capsules was at least 5% and greater than the incidence among placebo-treated patients. Incidence has been rounded to the nearest whole number.
Adverse Events With Other Marketed Methylphenidate Hydrochloride Products
Nervousness and insomnia are the most common adverse reactions reported with other methylphenidate products. Other reactions include hypersensitivity (including skin rash, urticaria, fever, arthralgia, exfoliative dermatitis, erythema multiforme with histopathological findings of necrotizing vasculitis, and thrombocytopenic purpura); anorexia; nausea; dizziness; palpitations; headache; dyskinesia; drowsiness; blood pressure and pulse changes, both up and down; tachycardia; angina; cardiac arrhythmia; abdominal pain; weight loss during prolonged therapy. There have been rare reports of Tourette’s Syndrome and obsessive-compulsive disorder. Toxic psychosis has been reported. Although a definite causal relationship has not been established, the following have been reported in patients taking this drug: instances of abnormal liver function, ranging from transaminase elevation to severe hepatic injury; isolated cases of cerebral arteritis and/or occlusion; leucopenia and/or anemia; transient depressed mood; a few instances of scalp hair loss. Very rare reports of neuroleptic malignant syndrome (NMS) have been reported, and, in most of these, patients were concurrently receiving therapies associated with NMS. In a single report, a ten year old boy who had been taking methylphenidate for approximately 18 months experienced an NMS-like event within 45 minutes of ingesting his first dose of venlafaxine. It is uncertain whether this case represented a drug-drug interaction, a response to either drug alone, or some other cause.
In children, loss of appetite, abdominal pain, weight loss during prolonged therapy, insomnia and tachycardia may occur more frequently; however, any of the other adverse reactions listed above may also occur.
Postmarketing Experience
In addition to the adverse events listed above, the following have been reported in patients receiving methylphenidate hydrochloride extended-release capsules worldwide. The list is alphabetized: abnormal behavior, aggression, anxiety, bruxism, cardiac arrest, depression, fixed drug eruption, hyperactivity, irritability, libido changes, migraine, obsessive-compulsive disorder, peripheral coldness, Raynaud’s phenomenon, reversible ischaemic neurological deficit, rhabdomyolysis, serotonin syndrome in combination with serotonergic drugs, sudden death, suicidal behavior (including completed suicide), and thrombocytopenia. Data are insufficient to support an estimation of incidence or establish causation.
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class
Methylphenidate hydrochloride extended-release capsules, like other methylphenidate products, are classified as a Schedule II controlled substance by federal regulation.
Abuse, Dependence, And Tolerance
See WARNINGS for boxed warning containing drug abuse and dependence information.
OVERDOSAGE
Signs And Symptoms
Signs and symptoms of acute methylphenidate overdosage, resulting principally from overstimulation of the CNS and from excessive sympathomimetic effects, may include the following: vomiting, agitation, tremors, hyperreflexia, muscle twitching, convulsions (may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, palpitations, cardiac arrhythmias, hypertension, mydriasis, dryness of mucous membranes, and rhabdomyolysis.
Recommended Treatment
Treatment consists of appropriate supportive measures. The patient must be protected against self-injury and against external stimuli that would aggravate overstimulation already present. Gastric contents may be evacuated by gastric lavage as indicated. Before performing gastric lavage, control agitation and seizures if present and protect the airway. Other measures to detoxify the gut include administration of activated charcoal and a cathartic. Intensive care must be provided to maintain adequate circulation and respiratory exchange; external cooling procedures may be required for hyperpyrexia.
Efficacy of peritoneal dialysis or extracorporeal hemodialysis for methylphenidate hydrochloride extended-release capsules overdosage has not been established.
The prolonged release of methylphenidate from methylphenidate hydrochloride extended-release capsules should be considered when treating patients with overdose.
DOSAGE AND ADMINISTRATION
Methylphenidate hydrochloride extended-release capsules are administered once daily in the morning, before breakfast.
Methylphenidate hydrochloride extended-release capsules may be swallowed whole with the aid of liquids, or alternatively, the capsule may be opened and the capsule contents sprinkled onto a small amount (tablespoon) of applesauce and given immediately, and not stored for future use. Drinking some fluids, e.g. water, should follow the intake of the sprinkles with applesauce. The capsules and the capsule contents must not be crushed or chewed (see PRECAUTIONS: Information for Patients). Patients should be advised to avoid alcohol while taking methylphenidate hydrochloride extended-release capsules.
Dosage should be individualized according to the needs and responses of the patient.
Initial Treatment
The recommended starting dose of methylphenidate hydrochloride extended-release capsules is 20 mg once daily. Dosage may be adjusted in weekly 10-20 mg increments to a maximum of 60 mg/day taken once daily in the morning, depending upon tolerability and degree of efficacy observed. Daily dosage above 60 mg is not recommended.
Maintenance/Extended Treatment
There is no body of evidence available from controlled trials to indicate how long the patient with ADHD should be treated with methylphenidate hydrochloride extended-release capsules. It is generally agreed, however, that pharmacological treatment of ADHD may be needed for extended periods. Nevertheless, the physician who elects to use methylphenidate hydrochloride extended-release capsules for extended periods in patients with ADHD should periodically re-evaluate the long-term usefulness of the drug for the individual patient with trials off medication to assess the patient’s functioning without pharmacotherapy. Improvement may be sustained when the drug is either temporarily or permanently discontinued.
Dose Reduction And Discontinuation
If paradoxical aggravation of symptoms or other adverse events occur, the dosage should be reduced, or, if necessary, the drug should be discontinued.
If improvement is not observed after appropriate dosage adjustment over a one-month period, the drug should be discontinued.
HOW SUPPLIED
Methylphenidate Hydrochloride Extended-release Capsules are available in six strengths:
10 mg, green cap and white body, opaque capsules, imprinted with CP over 401 on the cap and 10 mg on the body.
NDC: 64720-401-10 Bottle of 100 Capsules
20 mg, blue cap and white body, opaque capsules, imprinted with CP over 402 on the cap and 20 mg on the body.
NDC: 64720-402-10 Bottle of 100 Capsules
30 mg, brown cap and white body, opaque capsules, imprinted with CP over 403 on the cap and 30 mg on the body.
NDC: 64720-403-10 Bottle of 100 Capsules
40 mg, yellow cap and white body, opaque capsules, imprinted with CP over 404 on the cap and 40 mg on the body.
NDC: 64720-404-10 Bottle of 100 Capsules
50 mg, navy blue cap and white body, opaque capsules, imprinted with CP over 405 on the cap and 50 mg on the body.
NDC: 64720-405-10 Bottle of 100 Capsules
60 mg, white, opaque capsule imprinted with CP over 406 on the cap and 60 mg on the body.
NDC: 64720-406-10 Bottle of 100 Capsules
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
Keep out of the reach of children.
REFERENCE
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association 1994. 4th ed. Washington D.C.
LB# 871-01
Rev. March, 2017

Manufactured for and Distributed by:
CorePharma, LLC
Middlesex, NJ 08846
For additional copies of the printed patient information/medication guide, please visit www.corepharma.com or call 1-800-850-2719.
MEDICATION GUIDE
Methylphenidate Hydrochloride
(meth" il fen' i date hye" droe
klor' ide)
Extended-release Capsules CII
Read the Medication Guide that comes with methylphenidate hydrochloride extended-release capsules before you or your child starts taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about your or your child’s treatment with methylphenidate hydrochloride extended-release capsules.
|
What is the most important information I should know about methylphenidate hydrochloride extended-release capsules?
Tell your doctor if you or your child have any heart problems, heart defects, high blood pressure, or a family history of these problems.
Children and Teenagers
Tell your doctor about any mental problems you or your child have, or about a family history of suicide, bipolar illness, or depression.
|
What are methylphenidate hydrochloride extended-release capsules?
Methylphenidate hydrochloride extended-release capsules are a central nervous system stimulant prescription medicine. It is used for the treatment of Attention-Deficit Hyperactivity Disorder (ADHD).
Methylphenidate hydrochloride extended-release capsules may help increase attention and decrease impulsiveness and hyperactivity in patients with ADHD.
Methylphenidate hydrochloride extended-release capsules should be used as a part of a total treatment program for ADHD that may include counseling or other therapies.
| Methylphenidate hydrochloride extended-release capsules are a federally controlled substance (CII) because it can be abused or lead to dependence. Keep methylphenidate hydrochloride extended-release capsules in a safe place to prevent misuse and abuse. Selling or giving away methylphenidate hydrochloride extended-release capsules may harm others, and is against the law. Tell your doctor if you or your child have (or have a family history of) ever abused or been dependent on alcohol, prescription medicines or street drugs. |
Who should not take methylphenidate hydrochloride extended-release capsules?
Methylphenidate hydrochloride extended-release capsules should not be taken if you or your child:
- are very anxious, tense, or agitated
- have an eye problem called glaucoma
- have tics or Tourette’s syndrome, or a family history of Tourette’s syndrome. Tics are hard to control repeated movements or sounds.
- have severe high blood pressure or a heart problem
- have hyperthyroidism
- are taking or have taken within the past 14 days an antidepression medicine called a monoamine oxidase inhibitor or MAOI.
- are allergic to anything in methylphenidate hydrochloride extended-release capsules. See the end of this Medication Guide for a complete list of ingredients.
Methylphenidate hydrochloride extended-release capsules should not be used in children less than 6 years old because it has not been studied in this age group.
Methylphenidate hydrochloride extended-release capsules may not be right for you or your child. Before starting methylphenidate hydrochloride extended-release capsules tell your or your child’s doctor about all health conditions (or a family history of) including:
- heart problems, heart defects, high blood pressure
- mental problems including psychosis, mania, bipolar illness, or depression
- tics or Tourette’s syndrome
- seizures or have had an abnormal brain wave test (EEG)
- circulation problem in fingers and toes
Tell your doctor if you or your child is pregnant, planning to become pregnant, or breastfeeding.
Can methylphenidate hydrochloride extended-release capsules be taken with other medicines?
Tell your doctor about all of the medicines that you or your child take including prescription and nonprescription medicines, vitamins, and herbal supplements. Methylphenidate hydrochloride extended-release capsules and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted while taking methylphenidate hydrochloride extended-release capsules.
Your doctor will decide whether methylphenidate hydrochloride extended-release capsules can be taken with other medicines.
Especially tell your doctor if you or your child takes:
- anti-depression medicines including MAOIs
- seizure medicines
- blood thinner medicines
- blood pressure medicines
- cold or allergy medicines that contain decongestants
Know the medicines that you or your child takes. Keep a list of your medicines with you to show your doctor and pharmacist.
Do not start any new medicine while taking methylphenidate hydrochloride extended-release capsules without talking to your doctor first.
How should methylphenidate hydrochloride extended-release capsules be taken?
- Take methylphenidate hydrochloride extended-release capsules exactly as prescribed. Your doctor may adjust the dose until it is right for you or your child.
- Take methylphenidate hydrochloride extended-release capsules once each day in the morning before breakfast. Methylphenidate hydrochloride extended-release capsules are an extended release capsule. It releases medicine into your body throughout the day.
- Methylphenidate hydrochloride extended-release capsules can be taken with or without food.
- Swallow methylphenidate hydrochloride extended-release capsules whole with water or other liquids. If you cannot swallow the capsule, open it and sprinkle the medicine over a spoonful of applesauce. Swallow the applesauce and medicine mixture without chewing. Follow with a drink of water or other liquid. Never chew or crush the capsule or the medicine inside the capsule.
- Methylphenidate hydrochloride extended-release capsules should not be taken with alcohol. This may result in a more rapid release of the dose of methylphenidate hydrochloride extended-release capsules.
- From time to time, your doctor may stop methylphenidate hydrochloride extended-release capsules treatment for a while to check ADHD symptoms.
- Your doctor may do regular checks of the blood, heart, and blood pressure while taking methylphenidate hydrochloride extended-release capsules. Children should have their height and weight checked often while taking methylphenidate hydrochloride extended-release capsules. Methylphenidate hydrochloride extended-release capsules treatment may be stopped if a problem is found during these check-ups.
- If you or your child takes too much methylphenidate hydrochloride extended-release capsules or overdoses, call your doctor or poison control center right away, or get emergency treatment.
What are possible side effects of methylphenidate hydrochloride extended-release capsules?
See “What is the most important information I should know about methylphenidate hydrochloride extended-release capsules?” for information on reported heart and mental problems.
Other serious side effects include:
- slowing of growth (height and weight) in children
- seizures, mainly in patients with a history of seizures
- eyesight changes or blurred vision
- Painful and prolonged erections (priapism) have occurred with methylphenidate. If you or your child develop priapism, seek medical help right away. Because of the potential for lasting damage, priapism should be evaluated by a doctor immediately.
Common side effects include:
- headache
- decreased appetite
- stomach ache
- nervousness
- trouble sleeping
- dizziness
Talk to your doctor if you or your child has side effects that are bothersome or do not go away.
This is not a complete list of possible side effects. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store methylphenidate hydrochloride extended-release capsules?
Store methylphenidate hydrochloride extended-release capsules in a safe place at room temperature, 59 to 86°F (15 to 30°C). Protect from moisture.
Keep methylphenidate hydrochloride extended-release capsules and all medicines out of the reach of children.
General information about methylphenidate hydrochloride extended-release capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use methylphenidate hydrochloride extended-release capsules for a condition for which it was not prescribed. Do not give methylphenidate hydrochloride extended-release capsules to other people, even if they have the same condition. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about methylphenidate hydrochloride extended-release capsules. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about methylphenidate hydrochloride extended-release capsules that was written for healthcare professionals. For more information about methylphenidate hydrochloride extended-release capsules call 1-800-850-2719.
What are the ingredients in methylphenidate hydrochloride extended-release capsules?
Active Ingredient: methylphenidate hydrochloride, USP
Inactive Ingredients: sugar spheres, povidone, hydroxypropylmethylcellulose and polyethylene glycol, ethyl cellulose, cetyl alcohol, sodium lauryl sulfate, dibutyl sebacate, gelatin, and titanium dioxide.
The individual capsules contain the following coloring agents:
10 mg capsules: FD&C Blue No. 2, FDA/E172 Yellow Iron Oxide
20 mg capsules: D&C Red No. 28, FD&C Blue No. 1, FD&C Green No. 3
30 mg capsules: FDA/E172 Black Iron Oxide, FDA/E172 Red Iron Oxide, FDA/E172 Yellow Iron Oxide
40 mg capsules: D&C Yellow No. 10, FD&C Red No. 40
50 mg capsules: D&C Red No. 28, FD&C Green No. 3, FDA/E172 Black Iron Oxide
This Medication Guide has been approved by the U.S. Food and Drug Administration.
LB# 891
Rev. December, 2015

Manufactured for and Distributed by:
CorePharma, LLC
Middlesex, NJ 08846
For additional copies of the printed patient information/medication guide, please visit www.corepharma.com or call 1-800-850-2719.
PRINCIPAL DISPLAY PANEL
NDC: 64720-401-10
Once Daily
Methylphenidate
Hydrochloride
Extended-release
Capsules CII
10 mg
Rx Only
100 Capsules

PRINCIPAL DISPLAY PANEL
NDC: 64720-402-10
Once Daily
Methylphenidate
Hydrochloride
Extended-release
Capsules CII
20 mg
Rx Only
100 Capsules

PRINCIPAL DISPLAY PANEL
NDC: 64720-403-10
Once Daily
Methylphenidate
Hydrochloride
Extended-release
Capsules CII
30 mg
Rx Only
100 Capsules

PRINCIPAL DISPLAY PANEL
NDC: 64720-404-10
Once Daily
Methylphenidate
Hydrochloride
Extended-release
Capsules CII
40 mg
Rx Only
100 Capsules
PRINCIPAL DISPLAY PANEL
NDC: 64720-405-10
Once Daily
Methylphenidate
Hydrochloride
Extended-release
Capsules CII
50 mg
Rx Only
100 Capsules

PRINCIPAL DISPLAY PANEL
NDC: 64720-406-10
Once Daily
Methylphenidate
Hydrochloride
Extended-release
Capsules CII
60 mg
Rx Only
100 Capsules

| METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| METHYLPHENIDATE HYDROCHLORIDE
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - CorePharma, LLC (031192276) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.