Sinus Congestion PE by TOPCARE TCR-1131-2021-0426
Sinus Congestion PE by
Drug Labeling and Warnings
Sinus Congestion PE by is a Otc medication manufactured, distributed, or labeled by TOPCARE. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SINUS CONGESTION PE- phenylephrine hydrochloride tablet, coated
TOPCARE
----------
TCR-1131-2021-0426
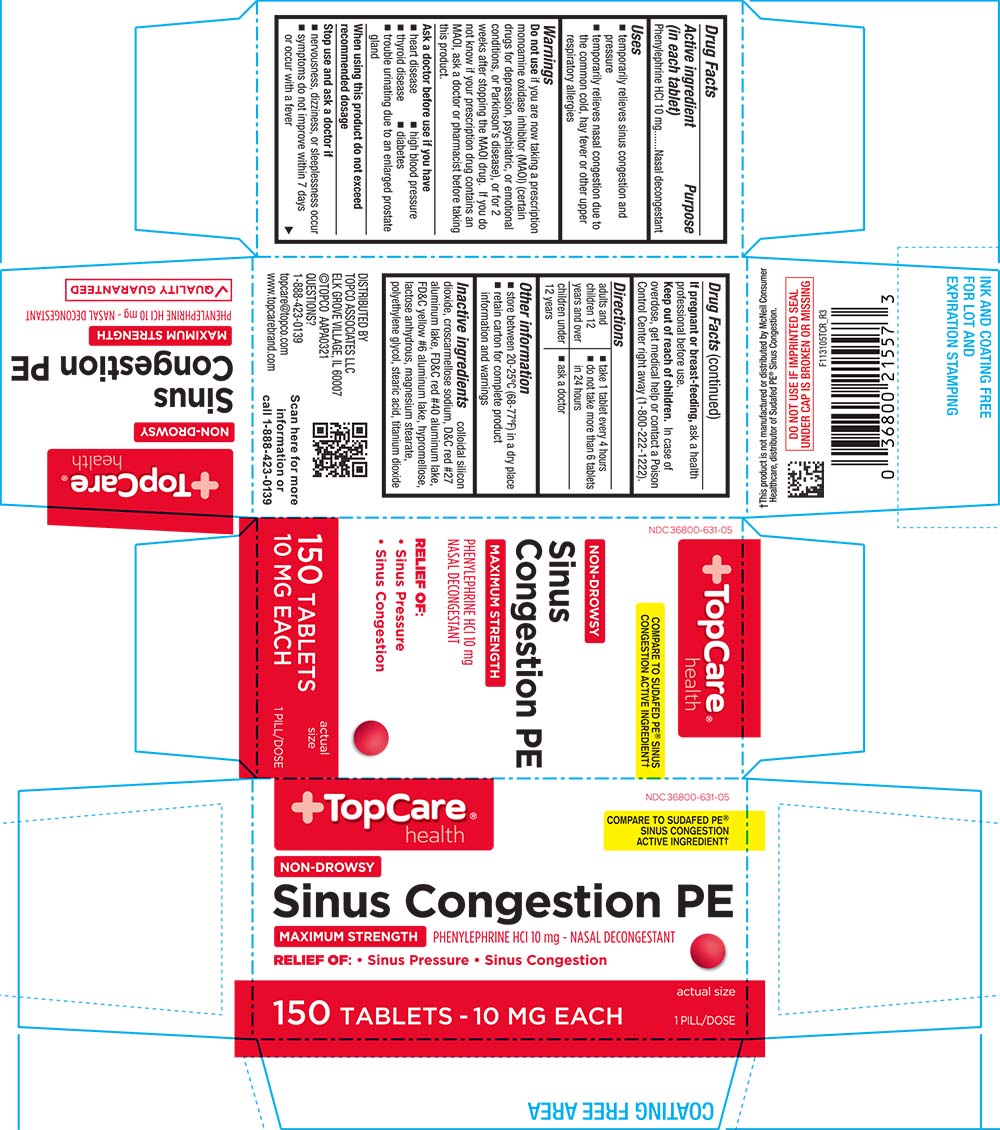
Uses
- temporarily relieves sinus congestion and pressure
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
Directions
- adults and children 12 years of age and over: take 1 tablet every 4 hours; do not take more than 6 tablets in 24 hours
- children under 12 years of age: ask a doctor
Other information
- store between 20-25°C (68-77°F) in a dry place
- retain carton for complete product information and warnings
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, D&C red #27 aluminum lake, FD&C red #40 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, lactose anhydrous, magnesium stearate, polyethylene glycol, stearic acid, titanium dioxide
PRINCIPAL DISPLAY PANEL
TopCare® health
NDC: 36800-631-05
COMPARE TO SUDAFED PE® SINUS CONGESTION ACTIVE INGREDIENT†
NON-DROWSY
Sinus Congestion PE
MAXIMUM STRENGTH
PHENYLEPHRINE HCl 10 mg - NASAL DECONGESTANT
RELIEF OF: Sinus Pressure Sinus Congestion
150 TABLETS - 10 MG EACH
actual size
1 PILL/DOSE
| SINUS CONGESTION PE
phenylephrine hydrochloride tablet, coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - TOPCARE (006935977) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.