TUMS SMOOTHIES- calcium carbonate tablet, chewable
TUMS by
Drug Labeling and Warnings
TUMS by is a Otc medication manufactured, distributed, or labeled by Haleon US Holdings LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (per tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug. Antacids may interact with certain prescription drugs.
- Directions
- Other information
- Inactive ingredients (Assorted Fruit)
- Inactive ingredients (Berry Fusion)
- Inactive ingredient (Assorted Tropical Fruit)
- Inactive ingredients (Peppermint)
- Questions?
-
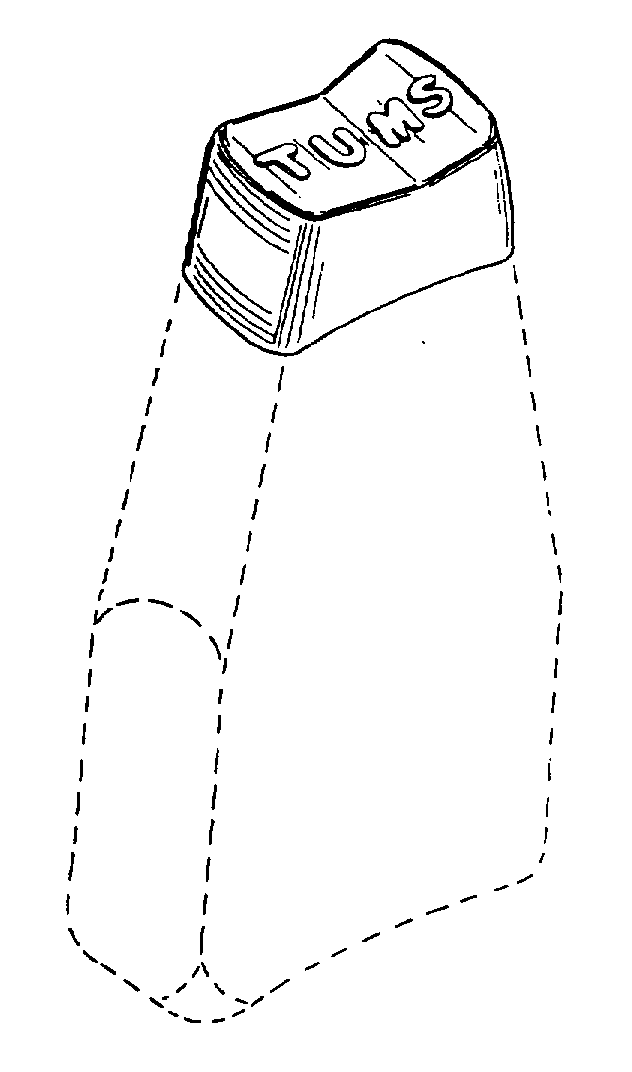
Principal Display Panel
NDC: 0135-0246-02
TUMS®
CALCIUM CARBONATE
ANTACID
SmoothiesTM
Assorted Fruit
SMOOTH DISSOLVE
EXTRA STRENGTH 750
GOES TO WORK IN SECONDS!
60 CHEWABLE TABLETS
PAREVE
©2014 GSK
Safety sealed – Do not use if printed inner seal beneath cap is missing or broken.
GlaxoSmithKline
Moon Twp, PA 15108
Gluten-Free
www.tums.com
Front Panel 103644XA
Back Panel 103553XA
-
Principal Display Panel
NDC: 0135-0456-03
TUMS®
CALCIUM CARBONATE
ANTACID
SmoothiesTM
Berry Fusion
SMOOTH DISSOLVE
EXTRA STRENGTH 750
GOES TO WORK IN SECONDS!
60 CHEWABLE TABLETS
PAREVE
Safety sealed – Do not use if printed inner seal beneath cap is missing or broken.
GSK CH
Warren, NJ 07059
Gluten-Free
©2014 GSK
Front Panel 103680XA
Back Panel 103725XA
-
Principal Display Panel
NDC: 0135-0245-02
TUMS®
CALCIUM CARBONATE
ANTACID
SmoothiesTM
Tropical Fruit
SMOOTH DISSOLVE
EXTRA STRENGTH 750
GOES TO WORK IN SECONDS!
60 CHEWABLE TABLETS
PAREVE
©2014 GSK
Safety sealed – Do not use if printed inner seal beneath cap is missing or broken.
GlaxoSmithKline
Moon Twp, PA 15108
Gluten-Free
www.tums.com
Front Panel 103818XA
Back Panel 103816XA
-
Principal Display Panel
NDC: 0135-0243-02
TUMS®
CALCIUM CARBONATE
ANTACID
SmoothiesTM
Peppermint
SMOOTH DISSOLVE
EXTRA STRENGTH 750
GOES TO WORK IN SECONDS!
60 CHEWABLE TABLETS
PAREVE
Safety sealed – Do not use if printed inner seal beneath cap is missing or broken.
Trademarks are owned by or licensed to the GSK group of companies.
GSK Consumer Healthcare
Warren, NJ 07059
Gluten-Free
©2015 GSK or its licensor.
Front Panel 103728XB
Back Panel 103727XB
-
INGREDIENTS AND APPEARANCE
TUMS SMOOTHIES
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0135-0246 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 750 mg Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) STARCH, CORN (UNII: O8232NY3SJ) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GUAR GUM (UNII: E89I1637KE) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SORBITOL (UNII: 506T60A25R) SUCROSE (UNII: C151H8M554) Product Characteristics Color PINK (orange, dark pink, yellow) Score no score Shape ROUND Size 19mm Flavor CHERRY (Assorted flavor, orange, strawberry, lemon) Imprint Code TUMS;SD Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0135-0246-01 12 in 1 CELLO PACK; Type 0: Not a Combination Product 03/24/2010 2 NDC: 0135-0246-02 60 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2010 3 NDC: 0135-0246-05 140 in 1 BOTTLE; Type 0: Not a Combination Product 08/10/2014 4 NDC: 0135-0246-07 250 in 1 BOTTLE; Type 0: Not a Combination Product 01/26/2016 5 NDC: 0135-0246-04 72 in 1 BOTTLE; Type 0: Not a Combination Product 03/14/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 03/24/2010 TUMS SMOOTHIES
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0135-0456 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 750 mg Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) STARCH, CORN (UNII: O8232NY3SJ) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) FD&C RED NO. 40 (UNII: WZB9127XOA) GUAR GUM (UNII: E89I1637KE) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SORBITOL (UNII: 506T60A25R) SUCROSE (UNII: C151H8M554) Product Characteristics Color PINK (mauve, peach, dark pink) Score no score Shape ROUND Size 19mm Flavor CHERRY (Berry Fusion, citrus berry, orange passion, strawberry) Imprint Code TUMS;SD Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0135-0456-01 12 in 1 CELLO PACK; Type 0: Not a Combination Product 03/24/2010 2 NDC: 0135-0456-03 60 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2010 3 NDC: 0135-0456-04 72 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2010 4 NDC: 0135-0456-05 140 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 03/24/2010 TUMS SMOOTHIES
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0135-0245 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 750 mg Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) STARCH, CORN (UNII: O8232NY3SJ) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GUAR GUM (UNII: E89I1637KE) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SORBITOL (UNII: 506T60A25R) SUCROSE (UNII: C151H8M554) Product Characteristics Color ORANGE (peach, mauve) Score no score Shape ROUND Size 19mm Flavor ORANGE (Assorted Tropical Fruit, orange passion, citrus berry, mixed fruit) Imprint Code TUMS;SD Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0135-0245-02 60 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 03/24/2010 TUMS SMOOTHIES
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0135-0243 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 750 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) GUAR GUM (UNII: E89I1637KE) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SORBITOL (UNII: 506T60A25R) SUCROSE (UNII: C151H8M554) Product Characteristics Color WHITE Score no score Shape ROUND Size 19mm Flavor PEPPERMINT Imprint Code TUMS;SD Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0135-0243-02 60 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 03/24/2010 Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263)
Trademark Results [TUMS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TUMS 98794008 not registered Live/Pending |
Haleon US IP LLC 2024-10-10 |
 TUMS 98058821 not registered Live/Pending |
BANG, Hai Hwa 2023-06-26 |
 TUMS 79373831 not registered Live/Pending |
BANG, Hai Hwa 2023-05-30 |
 TUMS 78226130 not registered Dead/Abandoned |
SmithKline Beecham Corporation 2003-03-17 |
 TUMS 77619345 3741016 Live/Registered |
GLAXOSMITHKLINE CONSUMER HEALTHCARE (US) IP LLC 2008-11-21 |
 TUMS 77155155 not registered Dead/Abandoned |
GLAXOSMITHKLINE LLC 2007-04-12 |
 TUMS 75696786 not registered Dead/Abandoned |
SmithKline Beecham Corporation 1999-05-03 |
 TUMS 75676521 2441507 Dead/Cancelled |
GLAXOSMITHKLINE LLC 1999-04-07 |
 TUMS 75465207 2240777 Dead/Cancelled |
SmithKline Beecham Corporation 1998-04-09 |
 TUMS 75058334 2076469 Live/Registered |
GLAXOSMITHKLINE CONSUMER HEALTHCARE (US) 1996-02-15 |
 TUMS 74723028 1979916 Live/Registered |
GLAXOSMITHKLINE CONSUMER HEALTHCARE (US) IP LLC 1995-08-30 |
 TUMS 74722865 1979915 Live/Registered |
GLAXOSMITHKLINE CONSUMER HEALTHCARE (US) IP LLC 1995-08-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.



