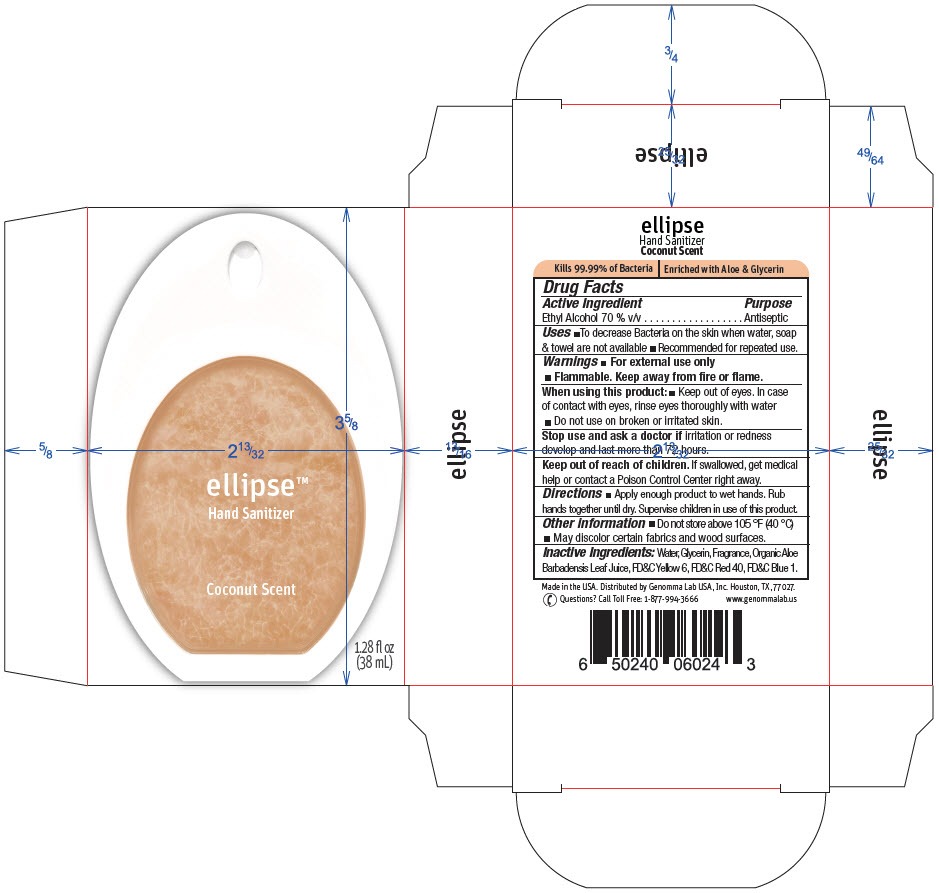
Ellipse™ Hand Sanitizer Coconut Scent
Ellipse Hand Sanitizer by
Drug Labeling and Warnings
Ellipse Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Genomma Lab USA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ELLIPSE HAND SANITIZER COCONUT SCENT- alcohol liquid
Genomma Lab USA
----------
Ellipse™ Hand Sanitizer
Coconut Scent
Uses
- To decrease Bacteria on the skin when water, soap & towel are not available
- Recommended for repeated use.
Warnings
- For external use only
- Flammable. Keep away from fire or flame.
Directions
- Apply enough product to wet hands. Rub hands together until dry. Supervise children in use of this product.
| ELLIPSE HAND SANITIZER
COCONUT SCENT
alcohol liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genomma Lab USA (832323534) |
Revised: 1/2025
Document Id: e2227998-5183-48af-a718-e96a8601b987
Set id: c22a7bbd-bd41-4ef4-ae58-0b0279b2f3dc
Version: 2
Effective Time: 20250131
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.