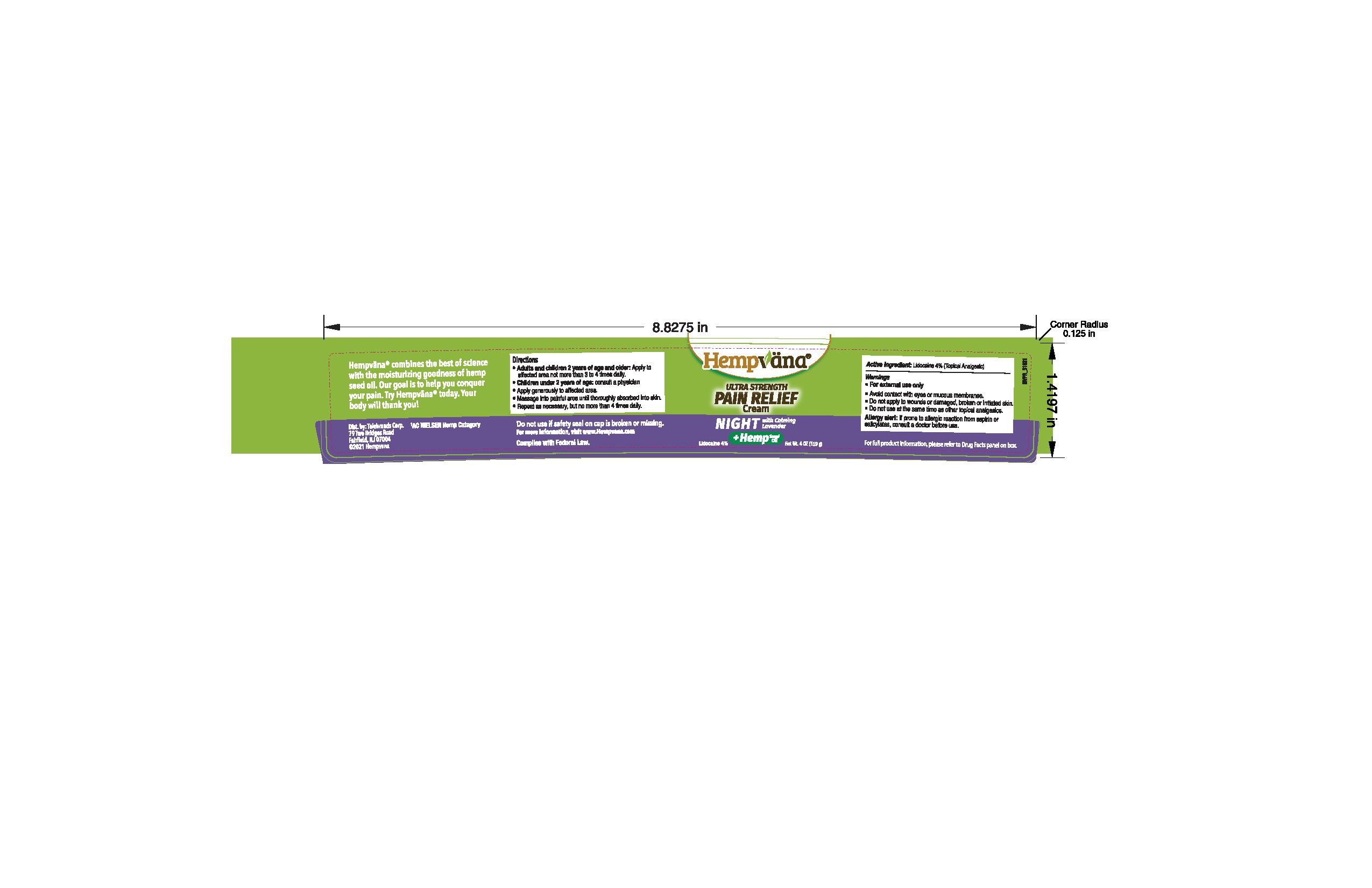
Hempvana Ultra Strength Pain Relief Cream - Night
Hempvana Ultra Strength Pain Relief Cream - Night by
Drug Labeling and Warnings
Hempvana Ultra Strength Pain Relief Cream - Night by is a Otc medication manufactured, distributed, or labeled by Telebrands Corp, Neutraderm, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HEMPVANA ULTRA STRENGTH PAIN RELIEF CREAM - NIGHT- lidocaine 4% cream
Telebrands Corp
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Hempvana Ultra Strength Pain Relief Cream - Night
Warnings
For external use only
Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
- Use only as directed. Read and follow all directions and warnings on this label.
- Do not bandage tightly or apply local heat (such as heating pads) to the area of use.
- Avoid contact with eyes or mucous membranes.
- Do not apply to wounds or damaged, broken or irritated skin.
- Do not use at the same time as other topical analgesics.
Directions
- For Use By Adults Only
- Apply generously to affected area
- Massage into painful area until thoroughly absorbed into skin.
- Repeat as necessary, but no more than 4 times daily.
Inactive Ingredients
Water/Aqua/Eau, Propylene Glycol, Ethylhexyl Palmitate, Dimethicone, Stearic Acid, Cannabis Sativa (Hemp) Seed Oil, Cetearyl Alcohol, Glyceryl Stearate, PEG-100 Stearate, Lavandula Angustifolia (Lavender) Oil, Melatonin, Piper Methysticum Root Extract, Anthemis Nobilis Flower Extract, Lavandula Angustifolia (Lavender) Flower Extract, Theobroma Cacao (Cocoa) Seed Butter, Potassium Cetyl Phosphate, Glycerin, Tocopheryl Acetate, Phenoxyethanol, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Caprylyl Glycol, Isohexadecane, Ethylhexylglycerin, Hexylene Glycol, Allantoin, Polysorbate 80, Disodium EDTA, Sodium Hydroxide, Red 4 (CI 14700), Blue 1 (CI 42090)
| HEMPVANA ULTRA STRENGTH PAIN RELIEF CREAM - NIGHT
lidocaine 4% cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Telebrands Corp (177266558) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Neutraderm, Inc. | 146224444 | manufacture(73287-017) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.