BISMUTH SUBCITRATE POTASSIUM, METRONIDAZOLE AND TETRACYCLINE HYDROCHLORIDE capsule
Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride by
Drug Labeling and Warnings
Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Par Pharmaceutical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BISMUTH SUBCITRATE POTASSIUM, METRONIDAZOLE AND TETRACYCLINE HYDROCHLORIDE CAPSULES safely and effectively. See full prescribing information for BISMUTH SUBCITRATE POTASSIUM, METRONIDAZOLE AND TETRACYCLINE HYDROCHLORIDE CAPSULES.
BISMUTH SUBCITRATE POTASSIUM, METRONIDAZOLE and TETRACYCLINE HYDROCHLORIDE capsules, for oral use
Initial U.S. Approval: 2006
WARNING: POTENTIAL FOR CARCINOGENICITY
See full prescribing information for complete boxed warning.
Metronidazole has been shown to be carcinogenic in mice and rats. It is unknown whether metronidazole is associated with carcinogenicity in humans (5.1).
RECENT MAJOR CHANGES
Warnings and Precautions, Severe Cutaneous Adverse Reactions (5.5) 4/2024
INDICATIONS AND USAGE
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules are a combination of metronidazole, a nitroimidazole antimicrobial, tetracycline, a tetracycline class antimicrobial and bismuth subcitrate potassium, indicated for use, in combination with omeprazole, for the treatment of patients with Helicobacter pylori infection and duodenal ulcer disease (active or history of within the past 5 years) to eradicate H. pylori. (1.1)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules and other antibacterial drugs, bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.2)
DOSAGE AND ADMINISTRATION
- Administer three bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules 4 times a day (after meals and at bedtime) for 10 days. (2)
- Administer bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules with omeprazole 20 mg twice daily (after the morning and evening meals). (2)
DOSAGE FORMS AND STRENGTHS
Each capsule of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules contains: (3)
- 140 mg of bismuth subcitrate potassium
- 125 mg metronidazole, USP
- 125 mg of tetracycline hydrochloride, USP
CONTRAINDICATIONS
- Concurrent usage of Methoxyflurane. (4.1, 7.1)
- Disulfiram usage within the last two weeks. (4.2, 7.2)
- Alcoholic beverage consumption for at least three days during or after therapy. (4.3, 7.3)
- Patients with Cockayne syndrome (4.4, 6.3)
- Severe renal impairment. (4.5)
- Women who are pregnant. (4.6, 8.1)
- Known hypersensitivity to product components. (4.7)
WARNINGS AND PRECAUTIONS
- Fetal Toxicity: Advise pregnant women of the risk throughout pregnancy for retardation of skeletal development seen in animal studies and permanent discoloration of teeth with tetracycline if used during the second or third trimester. (5.2, 8.1)
- Maternal Toxicity: Risk of hepatotoxicity in pregnant women with high doses of intravenous tetracycline also resulting in stillborn or premature birth. (5.3, 8.1)
- Tooth Enamel discoloration and hypoplasia: permanent discoloration may develop with use during tooth development (last half of pregnancy, infancy, and childhood to the age of 8 years). (5.4)
- Severe Cutaneous Adverse Reactions: Severe cutaneous adverse reactions (SCARs) including toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with metronidazole. If symptoms or signs of SCARs develop, discontinue bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride immediately and institute appropriate therapy. (5.5)
- Central and Peripheral Nervous System Effects: encephalopathy, convulsive seizures, aseptic meningitis and peripheral neuropathy with metronidazole, intracranial hypertension with tetracycline and neurotoxicity with bismuth-containing products. Monitor patients with CNS conditions closely and discontinue promptly if abnormal neurologic signs develop. (5.6)
- Photosensitivity: avoid exposure to sun and sun lamps. (5.8)
- Blood Dyscrasias: Use with caution in patients with a history of blood dyscrasias. (5.10)
- Hepatic Impairment: Not recommended in patients with severe hepatic impairment. (5.11)
ADVERSE REACTIONS
Most frequently reported adverse reactions (≥5%): abnormal feces, diarrhea, nausea, and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Endo at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Methoxyflurane: Risk of fatal renal toxicity; do not co-administer. (4.1, 7.1)
- Disulfiram: Psychotic reactions can occur; do not take concurrently or within the last 2 weeks of disulfiram. (4.2,7.2)
- Alcohol: Abdominal cramps, nausea, vomiting, headaches, and flushing can occur; do not consume during therapy and for at least 3 days afterwards. (4.3, 7.3)
- Oral Contraceptives: Decreased efficacy possibly resulting in pregnancy; use a different or additional form of contraception. (5.14, 7.4)
- Anticoagulants: Potentiation of the anticoagulant effect; Prothrombin time, International Normalized Ratio (INR), or other suitable anticoagulation tests should be closely monitored. (5.14, 7.5)
- Lithium: Increased lithium serum concentrations; measure serum lithium and serum creatinine concentrations during therapy. (5.14, 7.6)
- Antacids, Multivitamins or Dairy Products: Decreased absorption of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride; do not take concomitantly. (7.7)
- Busulfan: Increased busulfan serum concentrations; avoid concomitant use, monitor for busulfan toxicity. (7.8)
- CYP inducers and CYP inhibitors: Prolonged or accelerated half-life of metronidazole or concomitant medications; use with caution. (7.9, 7.10)
USE IN SPECIFIC POPULATIONS
- Lactation: A woman should pump and discard human milk for the duration of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride therapy, and for 2 days after therapy ends. (8.2)
- Pediatric Use: Tetracycline may cause permanent discoloration of the teeth. Enamel hypoplasia has also been reported. Do not use in children less than 8 years of age. (5.4, 8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: POTENTIAL FOR CARCINOGENICITY
1 INDICATIONS AND USAGE
1.1 Eradication of Helicobacter pylori in Patients with Active Duodenal Ulcer or History of Duodenal Ulcer Disease
1.2 Usage
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Methoxyflurane
4.2 Disulfiram
4.3 Alcohol
4.4 Cockayne Syndrome
4.5 Severe Renal Impairment
4.6 Pregnancy
4.7 Hypersensitivity Reactions
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Carcinogenicity
5.2 Fetal Toxicity
5.3 Maternal Toxicity
5.4 Tooth Enamel Discoloration and Hypoplasia
5.5 Severe Cutaneous Adverse Reactions
5.6 Central and Peripheral Nervous System Effects
5.7 Development of Potential for Microbial Overgrowth
5.8 Photosensitivity
5.9 Darkening of the Tongue and/or Black Stool
5.10 Use in Patients with Blood Dyscrasias
5.11 Increased Drug Plasma Concentrations in Patients with Hepatic Impairment
5.12 Laboratory Test Interactions
5.13 Development of Drug Resistant Bacteria
5.14 Drug Interactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
6.3 Other Important Adverse Reactions from Labeling for the Individual Components of Bismuth Subcitrate Potassium, Metronidazole And Tetracycline Hydrochloride
7 DRUG INTERACTIONS
7.1 Methoxyflurane
7.2 Disulfiram
7.3 Alcohol
7.4 Oral Contraceptives
7.5 Anticoagulants
7.6 Lithium
7.7 Antacids, Multivitamins, or Dairy Products
7.8 Busulfan
7.9 Inhibitors of CYP450 liver enzymes
7.10 Inducers of CYP450 liver enzymes
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Eradication of Helicobacter pylori in Patients with Active Duodenal Ulcer or History of Duodenal Ulcer Disease
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: POTENTIAL FOR CARCINOGENICITY
Metronidazole has been shown to be carcinogenic in mice and rats. It is unknown whether metronidazole is associated with carcinogenicity in humans [see Warning and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
1.1 Eradication of Helicobacter pylori in Patients with Active Duodenal Ulcer or History of Duodenal Ulcer Disease
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules in combination with omeprazole are indicated for the treatment of patients with Helicobacter pylori infection and duodenal ulcer disease (active or history of within the past 5 years) to eradicate H. pylori. The eradication of Helicobacter pylori has been shown to reduce the risk of duodenal ulcer recurrence.
1.2 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules and other antibacterial drugs, bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules should be used to treat only indicated infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
Administer three bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules 4 times a day (after meals and at bedtime) for 10 days. One omeprazole 20 mg capsule should be taken twice a day with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules after the morning and evening meal for 10 days (Table 1).
Table 1: Daily Dosing Schedule for Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules
Time of dose Number of capsules of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride Number of capsules of omeprazole 20 mg After morning meal 3 1 After lunch 3 0 After evening meal 3 1 At bedtime 3 0
Instruct patients to swallow the bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules whole with a full glass of water (8 ounces). Ingestion of adequate amounts of fluid, particularly with the bedtime dose, is recommended to reduce the risk of esophageal irritation and ulceration by tetracycline hydrochloride.If a dose is missed, patients should continue the normal dosing schedule until medication is gone. Patients should not take double doses. If more than 4 doses are missed, the prescriber should be contacted.
-
3 DOSAGE FORMS AND STRENGTHS
Each bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsule contains 140 mg of bismuth subcitrate potassium, 125 mg of metronidazole, USP and two smaller film coated tablets inside containing 125 mg of tetracycline hydrochloride, USP. The capsules are white opaque, size '0' hard gelatin capsule imprinted with “PAR” on cap and “440” on body.
-
4 CONTRAINDICATIONS
4.1 Methoxyflurane
Do not administer methoxyflurane to patients taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. The concurrent use of tetracycline hydrochloride, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, with methoxyflurane has been reported to result in fatal renal toxicity [see Drug Interactions (7.1)].
4.2 Disulfiram
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is contraindicated in patients who have taken disulfiram within the last two weeks. Psychotic reactions have been reported in alcoholic patients who are using metronidazole, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, and disulfiram concurrently [see Drug Interactions (7.2)].
4.3 Alcohol
Alcoholic beverages or other products containing propylene glycol should not be consumed during and for at least 3 days after therapy with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. A disulfiram-like reaction (abdominal cramps, nausea, vomiting, headaches, and flushing) may occur due to the interaction between alcohol or propylene glycol and metronidazole, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride [see Drug Interactions (7.3)].
4.4 Cockayne Syndrome
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is contraindicated in patients with Cockayne syndrome. Severe irreversible hepatotoxicity/acute liver failure with fatal outcomes have been reported after initiation of metronidazole in patients with Cockayne syndrome [see Adverse Reactions (6.3)].
4.5 Severe Renal Impairment
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is contraindicated in patients with severe renal impairment. The antianabolic action of the tetracyclines may cause an increase in blood urea nitrogen (BUN) [see Adverse Reactions (6.3)]. In patients with significantly impaired renal function, higher serum concentrations of tetracyclines may lead to azotemia, hyperphosphatemia, and acidosis.
4.6 Pregnancy
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is contraindicated during pregnancy [see Use in Specific Populations (8.1)].
4.7 Hypersensitivity Reactions
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is contraindicated in patients with known hypersensitivity (e.g. urticaria, erythematous rash, flushing, and fever) to bismuth subcitrate potassium, metronidazole or other nitroimidazole derivatives, or tetracycline [see Adverse Reactions (6.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Carcinogenicity
Metronidazole, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, has been shown to be carcinogenic in mice and rats. Tumors affecting the liver, lungs, mammary and lymphatic tissues have been detected in several studies of metronidazole in rats and mice, but not hamsters [see Nonclinical Toxicology (13)]. It is unknown whether metronidazole is associated with carcinogenicity in humans.
5.2 Fetal Toxicity
Tetracycline can cause fetal harm when administered to a pregnant woman. Based on animal data, use of drugs of the tetracycline class during the second and third trimester of pregnancy can cause permanent discoloration of the teeth (yellow-gray brown) and possibly inhibit bone development [see Warnings and Precautions (5.4)]. Administration of oral tetracycline to pregnant rats at various doses resulted in yellow fluorescence in teeth and bones in the newborn animals. If bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is used during pregnancy, or if the patient becomes pregnant while taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, advise the patient of the potential risk to the fetus [see Contraindications (4.6) and Use in Specific Populations (8.1)].
5.3 Maternal Toxicity
Tetracycline, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, administered during pregnancy at high doses (> 2 g IV) was associated with rare but serious cases of maternal hepatotoxicity. This syndrome may result in stillborn or premature birth due to maternal pathology [see Contraindications (4.6) and Use in Specific Populations (8.1)].
5.4 Tooth Enamel Discoloration and Hypoplasia
The use of drugs of the tetracycline class during tooth development (last half of pregnancy, infancy, and childhood to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown). This adverse reaction is more common during long-term use of the drug, but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, therefore, should not be used in this age group unless other drugs are not likely to be effective or are contraindicated [see Use in Specific Populations (8.4)].
5.5 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCARs) including toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with the use of metronidazole. Symptoms can be serious and potentially life threatening. If symptoms or signs of SCARs develop, discontinue bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules immediately and institute appropriate therapy.
5.6 Central and Peripheral Nervous System Effects
Metronidazole: Convulsive seizures, encephalopathy, aseptic meningitis and peripheral neuropathy (including optic neuropathy) have been reported. Encephalopathy has been reported in association with cerebellar toxicity characterized by ataxia, dizziness, and dysarthria. CNS lesions seen on MRI have been described in reports of encephalopathy. CNS symptoms are generally reversible within days to weeks upon discontinuation of metronidazole. CNS lesions seen on MRI have also been described as reversible. Peripheral neuropathy, mainly of sensory type has been reported and is characterized by numbness or paresthesia of an extremity. Aseptic meningitis symptoms may occur within hours of dose administration and generally resolve after metronidazole therapy is discontinued.
Tetracycline: Intracranial hypertension (IH), including pseudotumor cerebri, has been associated with the use of tetracyclines. Clinical manifestations of IH include headache, blurred vision, diplopia, and vision loss; papilledema can be found on fundoscopy. Women of childbearing age who are overweight or have a history of IH are at greater risk for developing tetracycline associated IH. Concomitant use of isotretinoin should be avoided because isotretinoin is also known to cause IH.
Although IH typically resolves after discontinuation of treatment, the possibility for permanent visual loss exists. If visual disturbance occurs during treatment, prompt ophthalmologic evaluation is warranted. Since intracranial pressure can remain elevated for weeks after drug cessation, patients should be monitored until they stabilize.
Bismuth-containing products: Cases of neurotoxicity associated with excessive doses of various bismuth-containing products have been reported. Effects have been reversible with discontinuation of bismuth therapy.
The appearance of abnormal neurologic signs and symptoms demands the prompt evaluation of the benefit/risk ratio of the continuation of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride therapy [see Adverse Reactions (6.3)].
5.7 Development of Potential for Microbial Overgrowth
Known or previously unrecognized candidiasis may present more prominent symptoms during therapy with metronidazole and requires treatment with an antifungal agent. As with other antibacterial drugs, use of tetracycline hydrochloride may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, discontinue bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride and institute appropriate therapy.
5.8 Photosensitivity
Photosensitivity, manifested by an exaggerated sunburn reaction, has been observed in patients taking tetracycline [see Adverse Reactions (6.3)]. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur with tetracycline drugs. Instruct patients taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride to avoid exposure to the sun or sun lamps. Discontinue treatment at the first evidence of skin erythema.
5.9 Darkening of the Tongue and/or Black Stool
Bismuth subcitrate potassium may cause temporary and harmless darkening of the tongue and/or black stools, generally reversible within several days after treatment is stopped [see Adverse Reactions (6.1)]. Stool darkening should not be confused with melena.
5.10 Use in Patients with Blood Dyscrasias
Metronidazole is a nitroimidazole, and should be used with care in patients with evidence of or history of blood dyscrasia. A mild leukopenia has been observed during its administration; however, no persistent hematologic abnormalities attributable to metronidazole have been observed in clinical studies. Total and differential leukocyte counts are recommended before and after therapy [see Adverse Reactions (6.3)].
5.11 Increased Drug Plasma Concentrations in Patients with Hepatic Impairment
Patients with hepatic impairment metabolize metronidazole slowly, with resultant accumulation of metronidazole in the plasma. Patients with mild to moderate hepatic impairment should be monitored for metronidazole associated adverse events. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is not recommended in patients with severe hepatic impairment (Child-Pugh C) [see Clinical Pharmacology (12.3)].
5.12 Laboratory Test Interactions
Bismuth absorbs x-rays and may interfere with x-ray diagnostic procedures of the gastrointestinal tract.
Bismuth subcitrate potassium may cause a temporary and harmless darkening of the stool. However, this change does not interfere with standard tests for occult blood.
Metronidazole may interfere with certain types of determinations of serum chemistry values, such as aspartate aminotransferase (AST, SGOT), alanine aminotransferase (ALT, SGPT), lactate dehydrogenase (LDH), triglycerides, and hexokinase glucose. Values of zero may be observed. All of the assays in which interference has been reported involve enzymatic coupling of the assay to oxidation-reduction of nicotinamide (NAD+ <=> NADH). Interference is due to the similarity in absorbance peaks of NADH (340 nm) and metronidazole (322 nm) at pH 7.
5.13 Development of Drug Resistant Bacteria
Prescribing bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.14 Drug Interactions
Oral Contraceptives
Concurrent use of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride with oral contraceptive may make oral contraceptives less effective due to an interaction with the tetracycline component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. Breakthrough bleeding has been reported. Advise women of child-bearing potential to use a different or additional form of contraception while taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride [see Drug Interactions (7.4)].
Anticoagulants
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride may alter the anticoagulant effects of warfarin and other oral coumarin anticoagulants. Metronidazole has been reported to potentiate the anticoagulant effect of warfarin, and other oral coumarin anticoagulants, resulting in a prolongation of prothrombin time. Tetracycline has been shown to depress plasma prothrombin activity. Closely monitor prothrombin time, International Normalized Ratio (INR), or other suitable anticoagulation tests if bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is administered concomitantly with warfarin. Patients should also be monitored for evidence of bleeding [see Drug Interactions (7.5)].
Lithium
In patients stabilized on relatively high doses of lithium, short-term use of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride may cause elevation of serum lithium concentrations and signs of lithium toxicity due to the interaction between metronidazole and lithium. Monitor serum lithium and serum creatinine concentrations daily for several days after beginning treatment with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride to detect any increase that may precede clinical symptoms of lithium toxicity [see Drug Interactions (7.6)].
Busulfan
Metronidazole has been reported to increase plasma concentrations of busulfan, which can result in an increased risk for serious busulfan toxicity. Do not administer bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride concomitantly with busulfan unless the benefit outweighs the risk. If no therapeutic alternatives to bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride are available, and concomitant administration with busulfan is medically needed, monitor for busulfan toxicity and busulfan plasma concentrations and adjust the busulfan dose accordingly [see Drug Interactions (7.8)].
Drugs that Prolong the QT interval
QT prolongation has been reported with metronidazole, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, particularly when administered with drugs with the potential for prolonging the QT interval.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride plus omeprazole (OBMT) to eradicate Helicobacter pylori was assessed in an open-label, randomized, active-controlled clinical trial conducted in North America. The duration of treatment was 10 days with 147 patients exposed to bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride plus omeprazole (OBMT) and 152 exposed to control, consisting of omeprazole, amoxicillin, and clarithromycin (OAC). The age of the population in the study ranged from 18 to 75 years, with 59% male patients and 59% Caucasian patients.
Adverse drug reactions were reported in 58% of patients in the OBMT group and 59% of patients in the OAC group. There were no adverse reactions leading to discontinuation of the study during the clinical trial.
Adverse reactions with an incidence of ≥ 5% in OBMT group include abnormal feces, diarrhea, nausea, and headache. Adverse drug reactions with an incidence of ≥ 5% in OAC group include diarrhea, dysgeusia, dyspepsia, nausea and headache.
Table 2 lists adverse reactions with an incidence of ≥ 1%, in either groups (OBMT vs OAC) and in order of decreasing incidence for the OBMT group.
Table 2: Adverse reactions with an incidence of ≥ 1% from North American trial, [n (%)]
Preferred Term OBMT* (n = 147) OAC** (n = 152) Gastrointestinal disorders Abnormal feces*** 23 (15.6%) 7 (4.6%) Nausea 12 (8.2%) 14 (9.2%) Diarrhea 10 (6.8%) 20 (13.2%) Abdominal Pain 7 (4.8%) 2 (1.3%) Dyspepsia 4 (2.7%) 10 (6.6%) Constipation 2 (1.4%) 5 (3.3%) Dry Mouth 2 (1.4%) 1 (0.7%) Flatulence 0 4 (2.6%) Glositis 0 2 (1.3%) General disorders and administration site conditions Asthenia 5 (3.4%) 2 (1.3%) Infections and infestations Vaginal infection 4 (2.7%) 3 (2%) Nervous system disorders Headache 8 (5.4%) 8 (5.3%) Dysgeusia 6 (4.1%) 18 (11.8%) Dizziness 4 (2.7%) 4 (2.6%) Investigations Laboratory test abnormal 3 (2%) 4 (2.6%) Alanine aminotransferase increased 2 (1.4%) 0 Aspartate aminotransferase increased 2 (1.4%) 0 Renal and urinary disorders Urine abnormality 2 (1.4%) 0 Skin and subcutaneous tissue disorders Rash Maculo-Papular 2 (1.4%) 0 Rash 1 (0.7%) 3 (2%) Pruritus 0 4 (2.6%) *OBMT = Omeprazole + Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride
**OAC = Omeprazole + Amoxicillin + Clarithromycin;
*** Dark stools [see Warnings and Precautions (5.9)]
Adverse reactions with an incidence of <1% for OBMT group are: back pain, vomiting, tongue darkening [see Warnings and Precautions (5.9)], anxiety, gastritis, gastroenteritis, myalgia, chest pain, increased appetite, blood creatine phosphokinase increased, malaise, somnolence, tachycardia, duodenal ulcer, visual disturbance, weight increased.
6.2 Postmarketing Experience
Additionally, the following adverse reactions, presented by system organ class in alphabetical order, have been identified during post approval use of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Gastrointestinal disorders: abdominal distention, eructation, flatulence
- General disorders and administration site conditions: chest discomfort, fatigue
- Infections and infestations: candidiasis, pseudomembranous colitis (Clostridium difficile colitis)
- Nervous Systems: peripheral neuropathy
- Skin and subcutaneous disorders: Stevens-Johnson syndrome, toxic epidermal necrolysis, DRESS syndrome (drug rash with eosinophilia and systemic symptoms) [see Warnings and Precautions (5.5)]
6.3 Other Important Adverse Reactions from Labeling for the Individual Components of Bismuth Subcitrate Potassium, Metronidazole And Tetracycline Hydrochloride
Metronidazole
Blood and Lymphatic system disorders: Reversible neutropenia (leucopenia) in cases of prolonged treatment; rarely reversible thrombocytopenia however no persistent hematological abnormalities attributable to metronidazole have been observed [see Warnings and Precautions (5.10)].
Cardiac disorders: QT prolongation has been reported with metronidazole, particularly when administered with drugs with the potential for prolonging the QT interval. Flattening of the T-wave may be seen in electrocardiographic tracings.
Gastrointestinal disorders: Nausea, vomiting, diarrhea, abdominal pain, constipation, anorexia, metallic taste, furry tongue, glossitis, stomatitis and candida overgrowth.
Hypersensitivity/Immune system disorders: Acute generalized exanthematous pustulosis (AGEP) [see Warnings and Precautions (5.5)], urticaria, erythematous rash, flushing, nasal congestion, dryness of the mouth (or vagina or vulva), and fever [see Contraindications (4.6)].
Metabolism and nutrition disorders: Pancreatitis.
Nervous system disorders: Convulsive seizures, encephalopathy, aseptic meningitis, optic and peripheral neuropathy, headache, syncope, dizziness, vertigo, incoordination, ataxia, tinnitus, hearing impairment, hearing loss, confusion, dysarthria, irritability, depression, weakness, and insomnia [see Warnings and Precautions (5.6)].
Dermatologic disorders: Erythematous rash and pruritus.
Renal and urinary disorders: Dysuria, cystitis, polyuria, incontinence, darkened urine, and a sense of pelvic pressure.
Hepatic: Cases of severe irreversible hepatotoxicity/acute liver failure, including cases with fatal outcomes with very rapid onset after initiation of systemic use of metronidazole, have been reported in patients with Cockayne Syndrome (latency from drug start to signs of liver failure as short as 2 days) [see Contraindications (4.4)].
Other: Dyspareunia, decrease of libido, proctitis, joint pains.
Tetracycline Hydrochloride
Blood and lymphatic system disorders: Hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, neutropenia, and eosinophilia.
Gastrointestinal disorders: Nausea, vomiting, diarrhea, anorexia, glossitis, black hairy tongue, dysphagia, enterocolitis, inflammatory lesions (with Candida overgrowth) in the anogenital region, esophagitis and esophageal ulceration.
Nervous system disorders: Intracranial hypertension including pseudotumor cerebri, tinnitus, and myasthenic syndrome.
Renal and urinary disorders: Increased BUN.
Skin and subcutaneous tissue disorders: Maculopapular and erythematous rashes, onycholysis, discoloration of the nails, exfoliative dermatitis and photosensitivity have been rarely reported [see Warnings and Precautions (5.8)].
Liver: Hepatotoxicity and liver failure.
Hypersensitivity reactions: Urticaria, angioedema, anaphylaxis, Henoch-Schonlein purpura, pericarditis, exacerbation of systemic lupus erythematosus, and serum sickness-like reactions.
-
7 DRUG INTERACTIONS
7.1 Methoxyflurane
Do not administer methoxyflurane to patients taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. The concurrent use of tetracycline hydrochloride, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, with methoxyflurane has been reported to result in fatal renal toxicity [see Contraindications (4.1)].
7.2 Disulfiram
Psychotic reactions have been reported in alcoholic patients who are using metronidazole, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride and disulfiram concurrently. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride should not be given to patients who have taken disulfiram within the last two weeks [see Contraindications (4.2)].
7.3 Alcohol
Consumption of alcoholic beverages or administration of other products containing propylene glycol during treatment with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride and for at least 3 days afterwards may cause a disulfiram-like reaction (abdominal cramps, nausea, vomiting, headaches, and flushing) due to the interaction between alcohol or propylene glycol and metronidazole, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. Discontinue alcoholic beverage or other products containing propylene glycol during and for at least 3 days after therapy with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride [see Contraindications (4.3)].
7.4 Oral Contraceptives
Concurrent use of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride with oral contraceptive may make oral contraceptives less effective due to an interaction with the tetracycline component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. Breakthrough bleeding has been reported. Women of child-bearing potential should use a different or additional form of contraception while taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride [see Warnings and Precautions (5.14)].
7.5 Anticoagulants
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride may alter the anticoagulant effects of warfarin and other oral coumarin anticoagulants. Metronidazole has been reported to potentiate the anticoagulant effect of warfarin, and other oral coumarin anticoagulants, resulting in a prolongation of prothrombin time. Tetracycline has been shown to depress plasma prothrombin activity. Prothrombin time, International Normalized Ratio (INR), or other suitable anticoagulation tests should be closely monitored if bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is administered concomitantly with warfarin. Patients should also be monitored for evidence of bleeding [see Warnings and Precautions (5.14)].
7.6 Lithium
In patients stabilized on relatively high doses of lithium, short-term use of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride may cause elevation of serum lithium concentrations and signs of lithium toxicity due to the interaction between metronidazole and lithium. Serum lithium and serum creatinine concentrations should be monitored several days after beginning treatment with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride to detect any increase that may precede clinical symptoms of lithium toxicity [see Warnings and Precautions (5.14)].
7.7 Antacids, Multivitamins, or Dairy Products
The absorption of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride may be reduced if administered with antacids containing aluminium, calcium, or magnesium; preparations containing iron, zinc, or sodium bicarbonate; or milk or dairy products due to the interaction between these products and tetracycline. These products should not be consumed concomitantly with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride. However, the clinical significance of reduced tetracycline systemic exposure is unknown as the relative contribution of systemic versus local antimicrobial activity against Helicobacter pylori has not been established.
7.8 Busulfan
Metronidazole has been reported to increase plasma concentrations of busulfan, which can result in an increased risk for serious busulfan toxicity. Do not administer bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride concomitantly with busulfan unless the benefit outweighs the risk. If no therapeutic alternatives to bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride are available, and concomitant administration with busulfan is medically needed, monitor for busulfan toxicity and busulfan plasma concentrations and adjust the busulfan dose accordingly [see Warnings and Precautions (5.14)].
7.9 Inhibitors of CYP450 liver enzymes
The simultaneous administration of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride and drugs that inhibit microsomal liver enzymes, such as cimetidine, may result in a prolonged half-life and decreased plasma clearance of metronidazole.
7.10 Inducers of CYP450 liver enzymes
The simultaneous administration of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride and drugs that induce microsomal liver enzymes, such as phenytoin or phenobarbital, may accelerate the elimination of metronidazole, resulting in reduced plasma concentrations of metronidazole. Impaired clearance of phenytoin has also been reported in this situation. Monitor phenytoin concentrations during treatment with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is contraindicated in women who are pregnant because treatment of Helicobacter pylori infection can be delayed in pregnant women, and the use of drugs of the tetracycline class during the second and third trimester pregnancy can also cause permanent discoloration of the teeth (yellow-gray brown) and possibly inhibit bone development [see Warnings and Precautions (5.2) and Data]. Administration of oral tetracycline to pregnant rats at various doses resulted in yellow fluorescence in teeth and bones in the newborn animals. There are maternal risks with high intravenous doses of tetracycline [see Clinical Considerations].
Metronidazole usage in pregnancy has been associated with certain congenital anomalies [see Data]. In animals, no fetotoxicity was observed when metronidazole was orally administered to pregnant mice at approximately 5% of the indicated human dose. There are no human or animal data on the use of bismuth subcitrate potassium during pregnancy. Although there are data on the separate components, there are no available data on the use of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride in pregnant women.
Clinical Considerations
Maternal Adverse Reactions
Tetracycline administered during pregnancy at high doses (> 2 g IV) was associated with rare but serious cases of maternal hepatotoxicity. This syndrome may result in stillborn or premature birth due to maternal pathology [see Warnings and Precautions (5.3)].
Data
Human Data
Tetracycline
Published case reports have described the yellowing of bones and teeth in human infants exposed to tetracycline during the second and third trimester of pregnancy. The yellowing is caused by the direct deposition of tetracycline during the mineralization process. This discoloration is more common during long-term use of the drug but has also been observed following repeated short-term courses. All tetracyclines form a stable calcium complex in any bone forming tissue. A decrease in fibula growth rate was observed in premature infants given oral tetracycline in doses of 25 mg/kg every six hours. The effect resolved when the drug was discontinued. One long-term follow-up study in children exposed to tetracycline in-utero showed no adverse effects on bone growth and development.
Metronidazole
There are published data from case-control studies, cohort studies, and 2 meta-analyses that include more than 5,000 pregnant women who used metronidazole during pregnancy. Many studies included first trimester exposures. One study showed an increased risk of cleft lip, with or without cleft palate, in infants exposed to metronidazole in-utero; however, these findings were not confirmed. In addition, more than ten randomized, placebo-controlled clinical trials enrolled more than 5,000 pregnant women to assess the use of antibiotic treatment (including metronidazole) for bacterial vaginosis on the incidence of preterm delivery. Most studies did not show an increased risk for congenital anomalies or other adverse fetal outcomes following metronidazole exposure during pregnancy. Three studies conducted to assess the risk of infant cancer following metronidazole exposure during pregnancy did not show an increased risk; however, the ability of these studies to detect such a signal was limited.
Bismuth subcitrate potassium
There are no human data on the use of bismuth subcitrate potassium during pregnancy.
Animal Data
Tetracycline
Results of animal studies indicate that tetracycline crosses the placenta, is found in fetal tissues, and can have toxic effects on the developing fetus (often related to reversible retardation of skeletal development). Evidence of embryotoxicity has also been noted in animals treated early in pregnancy. Multiple studies of limited design were conducted with pregnant and lactating female rats that resulted in fetuses and neonates with yellow discoloration of bones and teeth.
Metronidazole
Metronidazole crosses the placental barrier. No fetotoxicity was observed when metronidazole was administered orally to pregnant mice at 10 mg/kg/day, approximately 5 percent of the indicated human dose (1,500 mg/day) based on body surface area; however in a single small study where the drug was administered intraperitoneally, some intrauterine deaths were observed. The relationship of these findings to the drug is unknown.
Bismuth subcitrate potassium
Animal reproductive studies have not been conducted with bismuth subcitrate potassium.
8.2 Lactation
Risk Summary
Two of the individual components of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, tetracycline and metronidazole, are present in human milk at concentrations similar to maternal serum levels. It is not known whether bismuth subcitrate, the third component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is present in human milk. It is not known what effect metronidazole, tetracycline or bismuth has on the breastfed infant or on milk production. Tetracycline binds with calcium in human milk [see Clinical Pharmacology (12.3)]. Data indicate that oral absorption of tetracycline in infants is low due to the calcium binding in human milk. Metronidazole transfers to human milk, and infant serum levels can be close to or comparable to infant therapeutic levels. Because of the potential risk of tumorigenicity shown in animal studies with metronidazole, a woman should pump and discard human milk for the duration of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride therapy, and for 2 days after therapy ends, and feed her infant stored human milk (collected prior to therapy) or formula.
8.4 Pediatric Use
Safety and effectiveness of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride in pediatric patients infected with Helicobacter pylori have not been established.
Tetracycline use in children may cause permanent discoloration of the teeth. Enamel hypoplasia has also been reported. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride should not be used in children up to 8 years of age [see Warnings and Precaution (5.4)].
8.5 Geriatric Use
Clinical studies of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, elderly patients may have a greater frequency of decreased hepatic, renal, or cardiac function, and concomitant diseases or other drug therapies. Bismuth subcitrate potassium, a component of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, additional monitoring may be required [see Contraindications (4.5)].
8.6 Renal Impairment
The antianabolic action of the tetracyclines may cause an increase in blood urea nitrogen (BUN). In patients with severe renal impairment, higher serum concentrations of tetracycline may lead to azotemia, hyperphosphatemia, and acidosis [see Contraindications (4.5)].
8.7 Hepatic Impairment
Patients with severe hepatic disease metabolize metronidazole slowly, with resultant accumulation of metronidazole and its metabolites in plasma. Patients with mild to moderate hepatic impairment should be monitored for metronidazole associated adverse events. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is not recommended in patients with severe hepatic impairment [see Warnings and Precautions (5.11) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
In case of an overdose, patients should contact a physician, poison control center, or emergency room. The available overdosage information for each of the individual components in Bismuth subcitrate potassium, Metronidazole and Tetracycline hydrochloride are summarized below:
Metronidazole:
Single oral doses of metronidazole, up to 15 g, have been reported in suicide attempts and accidental overdoses. Symptoms reported include nausea, vomiting, and ataxia. Metronidazole is dialyzable.
Neurotoxic effects, including seizures and peripheral neuropathy, have been reported after 5 to 7 days of doses of 6 to 10.4 g every other day.
Treatment of Overdosage
There is no specific antidote for metronidazole overdose; therefore, management of the patient should consist of symptomatic and supportive therapy.
Tetracycline:
In case of overdosage, discontinue medication, treat symptomatically and institute supportive measures. Dialysis does not alter serum half-life and thus would not be of benefit in treating cases of overdosage.
Bismuth subcitrate potassium:
Symptoms of a bismuth subcitrate potassium overdosage are not known.
-
11 DESCRIPTION
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules are a combination antimicrobial product containing bismuth subcitrate potassium, metronidazole, and tetracycline hydrochloride for oral administration. Each size 0 elongated capsule contains:
- bismuth subcitrate potassium, 140 mg
- metronidazole, USP, 125 mg
- two smaller film coated tablets containing tetracycline hydrochloride, USP, 125 mg
Tetracycline hydrochloride is film coated to create a barrier to avoid contact with bismuth subcitrate potassium.
Each bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsule contains the following inactive ingredients: colloidal silicon dioxide, FD&C blue#2 aluminum lake, FD&C red#40 aluminum lake, gelatin, lecithin (soya), magnesium stearate, microcrystalline cellulose, polyvinyl alcohol, povidone, pregelatinized starch (maize), propylene glycol, shellac, sodium hydroxide, sodium lauryl sulphate, sodium stearyl fumarate, talc, titanium dioxide and xanthan gum.
Bismuth subcitrate potassium is a white to off white powder, hygroscopic in nature. It is a soluble, complex bismuth salt of citric acid. The schematized empirical molecular formula of bismuth subcitrate potassium is Bi (Citrate)2K5●3 H2O. The equivalent theoretical molecular formula is BiC12H14K5O17. The molecular mass of the theoretical molecular formula of a single unit of bismuth subcitrate potassium is 834.70.
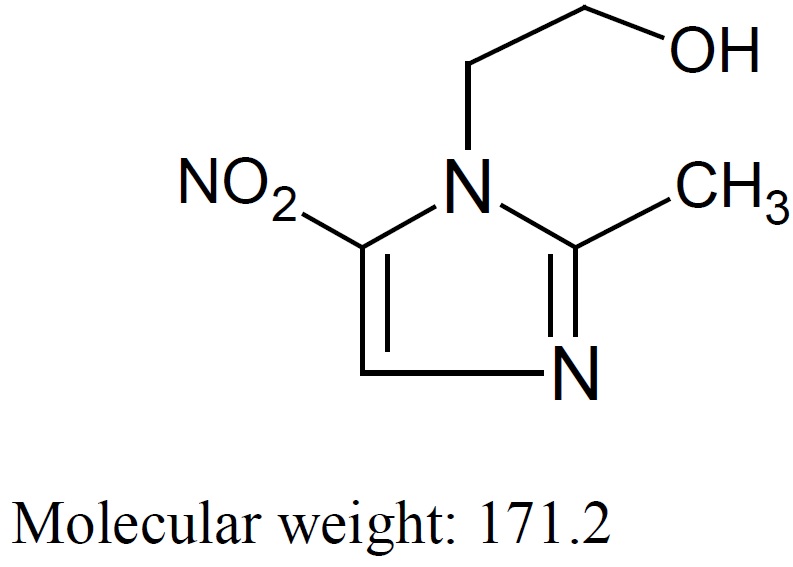
Metronidazole, USP is a white to pale yellow crystalline powder. Metronidazole, USP is 2-methyl-5-nitroimidazole-1-ethanol, with a molecular formula of C6H9N3O3 and the following structural formula:
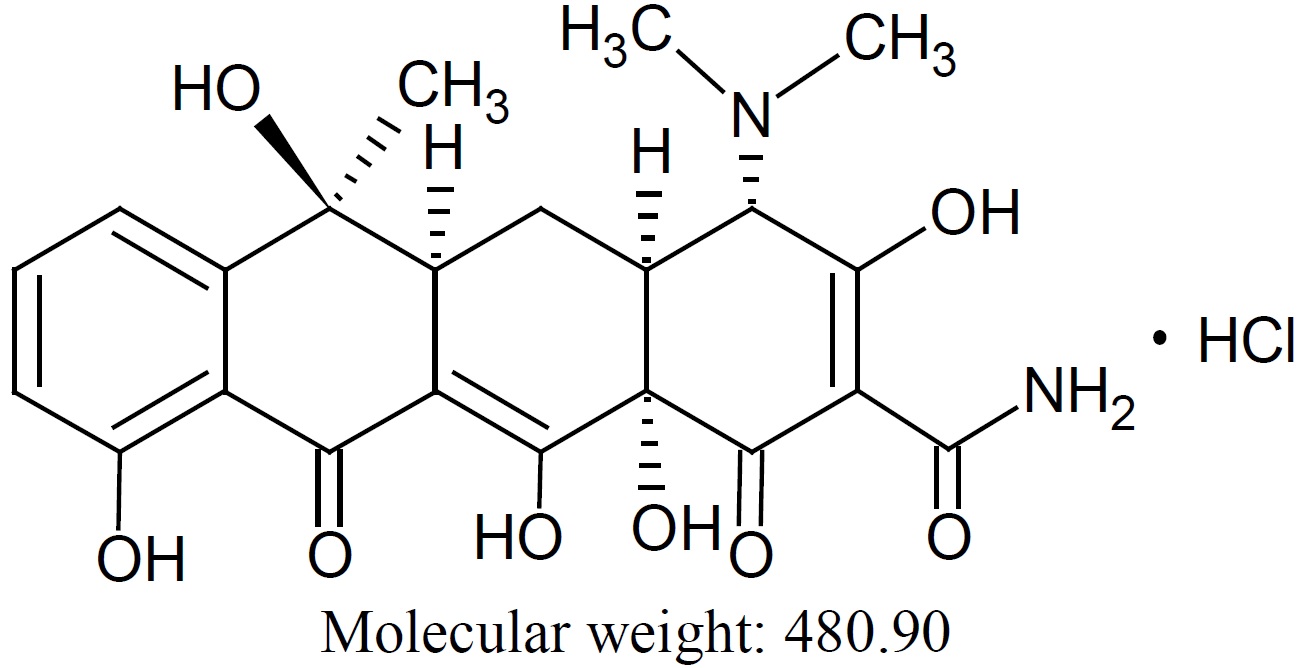
Tetracycline hydrochloride, USP is a yellow crystalline powder. Tetracycline hydrochloride, USP is stable in air, but exposure to strong sunlight causes it to darken. Tetracycline hydrochloride, USP is (4S,4aS,5aS,6S,12aS)-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacene-carboxamide monohydrochloride, with a molecular formula of C22H24N2O8●HCl and the following structural formula:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is a combination of antibacterial agents (metronidazole and tetracycline hydrochloride) and bismuth subcitrate potassium [see Microbiology (12.4)].
12.3 Pharmacokinetics
The pharmacokinetics of the individual components of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride are summarized below. In addition, two studies on bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride were conducted to determine the effect of co-administration on the pharmacokinetics of the components.
Bismuth Subcitrate Potassium (Bismuth)
Absorption and Distribution
Orally absorbed bismuth is distributed throughout the entire body. Bismuth is highly bound to plasma proteins (>90%).
Metabolism and Excretion
The elimination half-life of bismuth is approximately 5 days in both blood and urine. Elimination of bismuth is primarily through urinary and biliary routes. The rate of renal elimination appears to reach steady state 2 weeks after treatment discontinuation with similar rates of elimination at 6 weeks after discontinuation. The average urinary elimination of bismuth is 2.6% per day in the first two weeks after discontinuation (urine drug concentrations 24 to 250 mcg/mL) suggesting tissue accumulation and slow elimination.
Metronidazole
Absorption and Distribution
Following oral administration, metronidazole is well absorbed, with peak plasma concentrations occurring between 1 and 2 hours after administration. Plasma concentrations of metronidazole are proportional to the administered dose, with oral administration of 500 mg producing a peak plasma concentration of 12 mcg/mL.
Metronidazole appears in the plasma mainly as unchanged compound with lesser quantities of the 2-hydroxymethyl metabolite also present. Less than 20% of the circulating metronidazole is bound to plasma proteins. Metronidazole also appears in cerebrospinal fluid, saliva, and breast milk in concentration similar to those found in plasma.
Metabolism and Excretion
The average elimination half-life of metronidazole in normal volunteers is 8 hours. The major route of elimination of metronidazole and its metabolites is via the urine (60% to 80% of the dose), with fecal excretion accounting for 6% to 15% of the dose. The metabolites that appear in the urine result primarily from side-chain oxidation [1-(β-hydroxyethyl) 2-hydroxymethyl-5-nitroimidazole and 2-methyl-5-nitroimidazole-1-yl-acetic acid] and glucuronide conjugation, with unchanged metronidazole accounting for approximately 20% of the total. Renal clearance of metronidazole is approximately 10 mL/min/1.73m2.
Decreased renal function does not alter the single dose pharmacokinetics of metronidazole. In patients with decreased liver function, plasma clearance of metronidazole is decreased.
Tetracycline Hydrochloride
Absorption, Distribution, Metabolism and Excretion
Tetracycline hydrochloride is absorbed (60% to 90%) in the stomach and upper small intestine. The presence of food, milk or cations may significantly decrease the extent of absorption. In the plasma, tetracycline is bound to plasma proteins in varying degrees. It is concentrated by the liver in the bile and excreted in the urine and feces at high concentrations in biologically active form.
Tetracycline hydrochloride is distributed into most body tissues and fluids. It is distributed into the bile and undergoes varying degrees of enterohepatic recirculation. Tetracycline hydrochloride tends to localize in tumors, necrotic or ischemic tissue, liver and spleen and form tetracycline-calcium orthophosphate complexes at sites of new bone formation or tooth development. Tetracycline readily crosses the placenta and is excreted in high amounts in breast milk.
Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Capsules
A comparative bioavailability study of metronidazole (375 mg), tetracycline hydrochloride (375 mg) and bismuth subcitrate potassium (420 mg, equivalent to 120 mg Bi2O3) administered as bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride or as 3 separate capsule formulations administered simultaneously was conducted in healthy male volunteers. The pharmacokinetic parameters for the individual drugs, when administered as separate capsule formulations or as bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride, are similar as shown in Table 3.
Table 3: Mean (%CV) Pharmacokinetic Parameters for Metronidazole, Tetracycline hydrochloride, and Bismuth Subcitrate Potassium in Healthy Volunteers (N=18)
Cmax
(ng/mL)
(%C.V.**)AUCT
(ng h/mL)
(%C.V.**)AUC∞
(ng h/mL)
(%C.V.**)Metronidazole Metronidazole Capsule 9,044 (20) 80,289 (15) 81,849 (16) Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride* 8,666.3 (22) 83,018 (17) 84,413 (17) Tetracycline Tetracycline Capsules 748 (40) 9,544 (55) 9,864 (53) Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride* 774 (47) 9,674 (50) 9,987 (49) Bismuth Bismuth Capsule 22 (123) 47 (129) 65.4 (113) Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride* 17 (202) 43 (191) 57 (178) * Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride given as a single dose of 3 capsules
**C.V. – Coefficient Variation
Effect of Bismuth on the Bioavailability of Tetracycline Hydrochloride
There is an anticipated reduction in tetracycline hydrochloride systemic absorption due to an interaction with bismuth. The effect of a reduced tetracycline hydrochloride systemic exposure, due to an interaction with bismuth, on the clinical efficacy of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is not thought to be clinically meaningful as the contribution of systemic, as compared to local, antimicrobial activity against Helicobacter pylori has not been established.
Effect of Food on the Bioavailability of Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride
The pharmacokinetic parameters for metronidazole, tetracycline hydrochloride and bismuth were also determined when bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride was administered under fasting and fed conditions, as shown in Table 4. Food reduced the systemic absorption of all three bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride components, with AUC values for metronidazole, tetracycline hydrochloride and bismuth being reduced by 6%, 34% and 60%, respectively. Reduction in the absorption of all three bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride components in the presence of food is not considered to be clinically significant. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride should be given after meals and at bedtime, in combination with omeprazole twice a day.
Table 4: Mean Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Pharmacokinetic Parameters in Fasted and Fed States (N=18)*
FED FASTED metronidazole tetracycline bismuth metronidazole tetracycline bismuth Cmax
(ng/mL)
(%C.V.)
6,835
(13)515.8
(36)1.7
(61)8,666.3
(22)773.8
(47)16.7
(202)Tmax
(hours)**
(range)
3
(1.3 to 4)
4
(2.5 to 5)
3.5
(0.8 to 6)
0.75
(0.5 to 3.5)
3.3
(1.3 to 5)
0.6
(0.5 to 1.7)
AUC∞
(ng h/mL)
(%C.V.)79,225.6
(18)
5,840.1
(312)
18.4
(116)
84,413.6
(17)
9,986.7
(49)
56.5
(178)
* Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride given as a single dose of 3 capsules
**Tmax is expressed as median (range)
Effect of Omeprazole on the Bioavailability of Bismuth
The effect of omeprazole on bismuth absorption was assessed in 34 healthy volunteers given bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride (four times daily) with or without omeprazole (20 mg twice daily) for 6 days. In the presence of omeprazole, the extent of absorption of bismuth from bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride was significantly increased, compared to when no omeprazole was given (Table 5). Concentration-dependent neurotoxicity is associated with long-term use of bismuth and not likely to occur with short-term administration or at steady state concentrations below 50 ng/mL. One subject transiently achieved a maximum bismuth concentration (Cmax) higher than 50 ng/mL (73 ng/mL) following multiple dosing of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride with omeprazole. The patient did not exhibit symptoms of neurotoxicity during the study. There is no clinical evidence to suggest that short-term exposure to bismuth Cmax concentrations above 50 ng/mL is associated with neurotoxicity.
Table 5: Mean Bismuth Pharmacokinetic Parameters following Bismuth Subcitrate Potassium, Metronidazole and Tetracycline Hydrochloride Administration* With and Without Omeprazole (N=34)
Parameter Without omeprazole With omeprazole Mean %C.V.** Mean %C.V.** Cmax (ng/mL) 8.1 84 25.5 69 AUCT (ng h/mL) 48.5 28 140.9 42 * Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride given as 3 capsules four times daily for 6 days with or without 20 mg omeprazole twice daily
**C.V. – Coefficient Variation
12.4 Microbiology
Mechanism of Action
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride is a combination of antibacterial agents (metronidazole and tetracycline hydrochloride) and bismuth subcitrate potassium.
Tetracycline hydrochloride interacts with the 30S subunit of the bacterial ribosome and inhibits protein synthesis. Metronidazole’s antibacterial mechanism of action in an anaerobic environment is not fully understood but a possible mechanism includes reduction by intracellular electron transport proteins after entry into the organism. Because of this alteration to the metronidazole molecule, a concentration gradient is created and maintained which promotes the drug’s intracellular transport. Presumably, free radicals are formed which, in turn, react with cellular components resulting in death of bacteria. The antibacterial action of bismuth salts is not well understood.
Antimicrobial Activity
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride plus omeprazole therapy has been shown to be active against most isolates of Helicobacter pylori both in vitro and in clinical infections [see Clinical Studies (14)].
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies have been performed to evaluate the effect of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride on carcinogenesis, mutagenesis, or impairment of fertility.
Bismuth Subcitrate Potassium
No carcinogenicity or reproductive toxicity studies have been conducted with bismuth subcitrate potassium. Bismuth subsalicylate did not show mutagenic potential in the NTP Salmonella plate assay.
Metronidazole
Metronidazole has shown evidence of carcinogenic activity in a number of studies involving chronic, oral administration in mice and rats. Prominent among the effects in the mouse was an increased incidence of pulmonary tumorigenesis. This has been observed in all six reported studies in that species, including one study in which the animals were dosed on an intermittent schedule (administration during every fourth week only). At the highest dose levels, (approximately 500 mg/kg/day, which is approximately 1.6 times the indicated human dose for a 60 kg adult based on body surface area) there was a statistically significant increase in the incidence of malignant liver tumors in male mice. Also, the published results of one of the mouse studies indicate an increase in the incidence of malignant lymphomas as well as pulmonary neoplasms associated with lifetime feeding of the drug. All these effects are statistically significant. Long-term, oral-dosing studies in the rat showed statistically significant increases in the incidence of various neoplasms, particularly in mammary and hepatic tumors, among female rats administered metronidazole over those noted in the concurrent female control groups. Two lifetime tumorigenicity studies in hamsters have been performed and reported to be negative. Although metronidazole has shown mutagenic activity in a number of in vitro assay systems, studies in mammals (in vivo) have failed to demonstrate a potential for genetic damage.
Fertility studies have been conducted with male rats and mice with divergent results. Metronidazole, at doses up to 400 mg/kg/day (approximately 3 times the indicated human dose based on mg/m2) for 28 days, failed to produce any adverse effects on fertility and testicular function in male rats. Rats treated with up to 400 mg/kg/day for 6 weeks or longer, showed severe degeneration of the seminiferous epithelium in the testes which was associated with a marked decrease in testicular spermatid counts and epididymal sperm counts and a marked decrease in fecundity. These effects were partially reversible.
Fertility studies have been performed in male mice at doses up to six times the maximum recommended human dose based upon mg/m2 and have revealed no evidence of impaired fertility. Another fertility study was performed in male mice at oral doses of 500 mg/kg/day (approximately 2 times the indicated human dose based on mg/m2) for 14 days. Metronidazole significantly decreased testes and epididymides weight, decreased sperm viability, and increased the incidence of abnormal sperm. The viability of sperm was normal by 2 months after the start of the treatment.
Tumors affecting the liver, lungs, mammary, and lymphatic tissues have been detected in several studies of metronidazole in rats and mice, but not hamsters.
Pulmonary tumors have been observed in all six reported studies in the mouse, including one study in which the animals were dosed on an intermittent schedule (administration during every fourth week only). Malignant liver tumors were increased in male mice treated at approximately 1,500 mg/m2 (similar to the maximum recommended daily dose, based on body surface area comparisons).
Malignant lymphomas and pulmonary neoplasms were also increased with lifetime feeding of the drug to mice. Mammary and hepatic tumors were increased among female rats administered oral metronidazole compared to concurrent controls. Two lifetime tumorigenicity studies in hamsters have been performed and reported to be negative.
Metronidazole has shown mutagenic activity in in vitro assay systems including the Ames test. Studies in mammals in vivo have failed to demonstrate a potential for genetic damage.
Tetracycline hydrochloride
There has been no evidence of carcinogenicity for tetracycline hydrochloride in studies conducted with rats and mice. Some related antibiotics (oxytetracycline, minocycline) have shown evidence of oncogenic activity in rats.
There was evidence of mutagenicity by tetracycline hydrochloride in two in vitro mammalian cell assay systems (L51784y mouse lymphoma and Chinese hamster lung cells).
Tetracycline hydrochloride had no effect on fertility when administered in the diet to male and female rats at a daily intake of 25 times the human dose.
-
14 CLINICAL STUDIES
14.1 Eradication of Helicobacter pylori in Patients with Active Duodenal Ulcer or History of Duodenal Ulcer Disease
An open-label, parallel group, active-controlled, multicenter study in Helicobacter pylori positive patients with current duodenal ulcer or a history of duodenal ulcer disease was conducted in the United States and Canada (the North American Study).
Patients were randomized to one of the following 10-day treatment regimens:
- Three (3) bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules four times daily, after meals and at bedtime plus 20 mg omeprazole twice a day after the morning and evening meals (OBMT).
- Clarithromycin 500 mg plus 1,000 mg amoxicillin plus 20 mg omeprazole twice a day before the morning and evening meals (OAC).
H. pylori eradication rates, defined as two negative 13C-urea breath tests performed at 4 and 8 weeks post-therapy are shown in Table 6 for OBMT and OAC. The eradication rates for both groups were found to be similar using either the Per Protocol (PP) or Modified Intent-to-Treat (MITT) populations.
Table 6: Helicobacter pylori Eradication at 8 Weeks after 10 Day Treatment Regimen Percent (%) of Patients Cured [95% Confidence Interval] (Number of Patients)
Treatment Group Difference OBMT* OAC** c Per Protocola 92.5%
[87.8, 97.2]
(n=120)85.7%
[76.9, 91.8]
(n=126)6.8%
[-0.9, 14.5]Modified
Intent-to-Treatb87.7%
[82.2, 93.2]
(n=138)83.2%
[77, 89.5]
(n=137)4.5%
[-3.9, 12.8]*OBMT: Omeprazole + bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride
**OAC: Omeprazole + amoxicillin + clarithromycin
aPatients were included in the PP analysis if they had H. pylori infection documented at baseline, defined as a positive 13C-UBT plus histology or culture, had at least one endoscopically verified duodenal ulcer ≥ 0.3 cm at baseline or had a documented history of duodenal ulcer disease, and were not protocol violators. Additionally, if patients dropped out of the study due to an adverse event related to the study drug, they were included in the evaluable analysis as failures of therapy.
bPatients were included in the MITT analysis if they had documented H. pylori infection at baseline as defined above, and had at least one documented duodenal ulcer at baseline or had a documented history of duodenal ulcer disease, and took at least one dose of study medication. All dropouts were included as failures of therapy.
cResults for OAC treatment represent all isolates regardless of clarithromycin susceptibility. Eradication rates for clarithromycin susceptible organisms, as defined by an MIC ≤ 0.25 mcg/mL, were 94.6% and 92.1% for the PP and MITT analysis, respectively. Eradication rates for clarithromycin non-susceptible organisms, as defined by an MIC ≥ 0.5 mcg/mL, were 23.1% and 21.4% for the PP and MITT analysis, respectively.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules, 140 mg/125 mg/125 mg are supplied as a white opaque capsule containing 140 mg bismuth subcitrate potassium, 125 mg metronidazole, USP, and 125 mg tetracycline hydrochloride, USP, with the "440" printed on the body and "PAR" printed on the cap. Bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules are supplied as the 10 day Therapy pack containing 10 blister cards, with each card containing 12 capsules for a total of 120 capsules.
Blister pack of 12 Capsules NDC: 49884-151-24
Carton of 120 Capsules NDC: 49884-151-54
Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Lactation
Advise the lactating women to pump and discard their milk during treatment with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules and for 2 days after the therapy ends [see Use in Specific Populations (8.2)].
Hypersensitivity
Inform patients that bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules may cause allergic reactions and to discontinue bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules at the first sign of urticaria, erythematous rash, flushing, and fever or other symptoms of an allergic reaction [see Contraindications (4.7)].
Severe Cutaneous Adverse Reactions
Advise patients that bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules may increase the risk of serious and sometimes fatal dermatologic reactions, including toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), and drug reaction with eosinophilia and systemic symptoms (DRESS). Instruct the patient to be alert for skin rash, blisters, fever or other signs and symptoms of these hypersensitivity reactions. Advise patients to stop bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules immediately if they develop any type of rash and seek medical attention [see Warnings and Precautions (5.5)].
Central Nervous System Effects
Inform patients of the risk of central and peripheral nervous system effects with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules and to discontinue bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules and report immediately to their health-care provider if any neurologic symptoms occur [see Warnings and Precautions (5.6)].
Photosensitivity
Avoid exposure to sun or sun lamps while taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules [see Warnings and Precautions (5.8)].
Drug Interactions
Advise patients to report to their health-care provider the use of any other medications while taking bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules. The administration of any of the following drugs with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules may result in clinically significant adverse reactions or insufficient drug efficacies [see Contraindications (4) and Drug Interactions (7)]:
- Methoxyflurane
- Disulfiram
- Alcoholic Beverages, or Products Containing Propylene Glycol
- Oral Contraceptives
- Anticoagulants
- Lithium
- Antacids, Multivitamins, or Dairy Products
- Busulfan
- Cimetidine
- Phenytoin and Phenobarbital
Darkening of the Tongue and/or Stool
Inform patients that bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules may cause temporary and harmless darkening of the tongue and/or black stool generally reversible within several days after treatment is stopped. Stool darkening should not be confused with melena (blood in the stool) [see Warnings and Precautions (5.9)].
Dosing Information
Inform patients that each dose of bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules includes 3 capsules. All 3 capsules should be taken 4 times a day (after meals and at bedtime) for 10 days. One omeprazole 20 mg capsule should be taken twice a day with bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules after the morning and evening meal for 10 days.
If a dose is missed, advise patient not to make up the dose, but to continue the normal dosing schedule until medication is gone.
Patients should not take double doses. If more than 4 doses are missed, advise the patient to contact their health-care provider [see Dosage and Administration (2)].
Administration with Fluids
Instruct patients to swallow the bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules whole with a full glass of water (8 ounces). Ingestion of adequate amounts of fluid, particularly with the bedtime dose, is recommended to reduce the risk of esophageal irritation and ulceration by tetracycline hydrochloride [see Dosage and Administration (2)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs including bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsules or other antibacterial drugs in the future.
Manufactured for:
Endo USA
Malvern, PA 19355 U.S.A.
Made in India
Neutral Code: TN/DRUGS/TN00002122
© 2024 Endo, Inc. or one of its affiliates.
OS151-01-74-03
Revised: 12/2024
- PACKAGE LABEL. PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BISMUTH SUBCITRATE POTASSIUM, METRONIDAZOLE AND TETRACYCLINE HYDROCHLORIDE
bismuth subcitrate potassium, metronidazole and tetracycline hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 49884-151 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISMUTH SUBCITRATE POTASSIUM (UNII: R3O80H60KX) (BISMUTH CATION - UNII:ZS9CD1I8YE) BISMUTH SUBCITRATE POTASSIUM 140 mg METRONIDAZOLE (UNII: 140QMO216E) (METRONIDAZOLE - UNII:140QMO216E) METRONIDAZOLE 125 mg TETRACYCLINE HYDROCHLORIDE (UNII: P6R62377KV) (TETRACYCLINE - UNII:F8VB5M810T) TETRACYCLINE HYDROCHLORIDE 125 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE (UNII: FZ989GH94E) SHELLAC (UNII: 46N107B71O) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white (white opaque) Score no score Shape CAPSULE Size 21mm Flavor Imprint Code PAR;440 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49884-151-54 120 in 1 CARTON; Type 0: Not a Combination Product 03/07/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205770 03/07/2023 Labeler - Endo USA, Inc. (119185057)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

