PLUM EMERGENCY EYEWASH/EYEWASH DUO/EYEWASH SHOWER ISS USA- water liquid
Plum Emergency Eyewash/Eyewash DUO/Eyewash Shower ISS USA by
Drug Labeling and Warnings
Plum Emergency Eyewash/Eyewash DUO/Eyewash Shower ISS USA by is a Otc medication manufactured, distributed, or labeled by Plum A/S, Holopack Verpackungstechnik GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Ask doctor if you have
- Keep out of reach of children
-
Directions
- do not dilute solution or reuse bottle
- use only unopened bottle
- to open, twist cap in the direction of the arrow
- avoid contamination of the integrated eyecup
- place integrated eyecup over affected eye(s)
- tilt head backward
- open eyelid(s) wide
- control rate of flow by pressure on bottle
- thoroughly bathe eye(s) with solution
- allow solution to flow away from eye(s)
- rinse until the bottle is empty and continue rinsing with Plum Emergency Eyewash until you reach a doctor
- continue rinsing with water if necessary
- obtain medical treatment.
- Other Information
- Inactive ingredients
- Questions or Comments
- Imported and distributed by
-
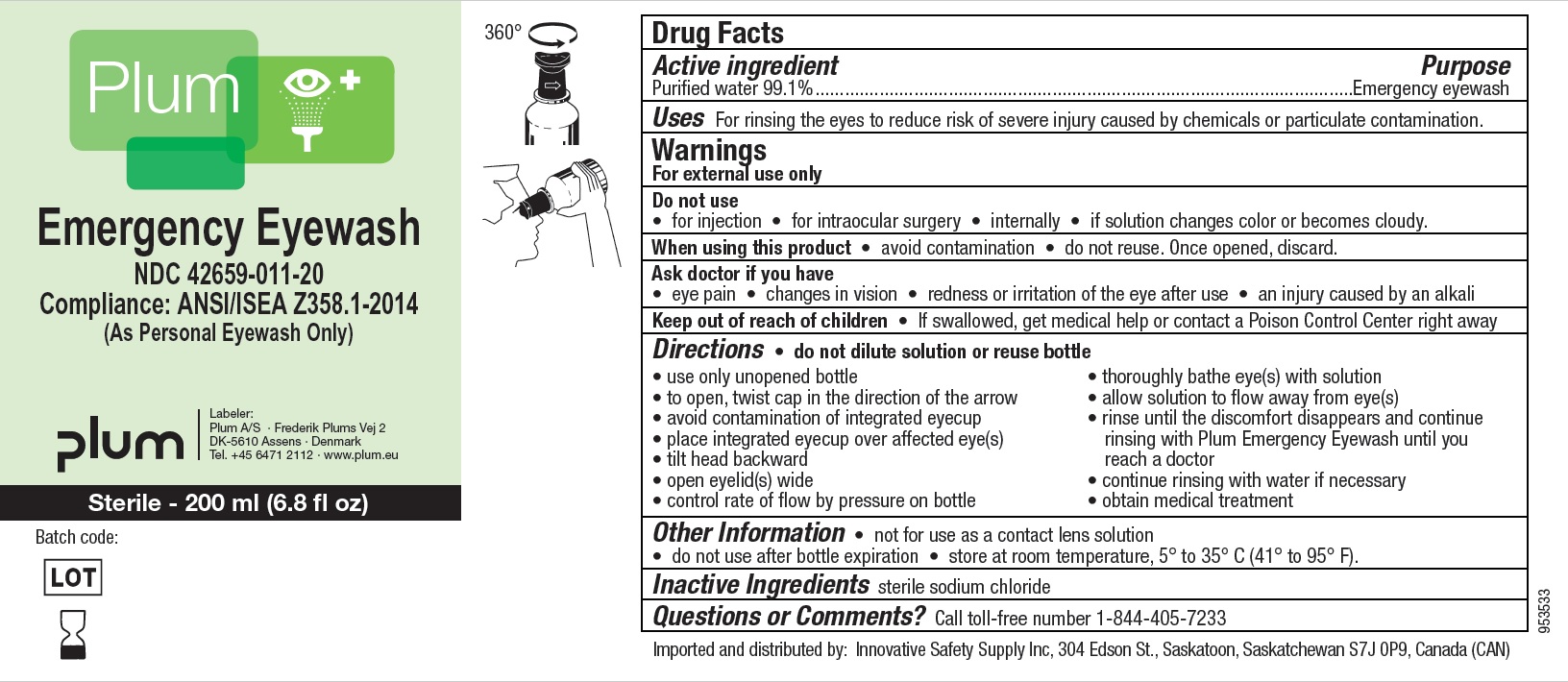
Principal Display Panel
NDC : 42659-011-20
Plum
Emergency Eyewash
Compliance: ANSI/ISEA Z358.1-2014
(As Personal Eyewash Only)
plum
Labeler: Plum A/S - Frederik plums Vej 2
DK - 5610 Assens - Denmark
Tel. +45 6471 2112
www.plum.eu
Sterile - 200 ml (6.8 fl oz)
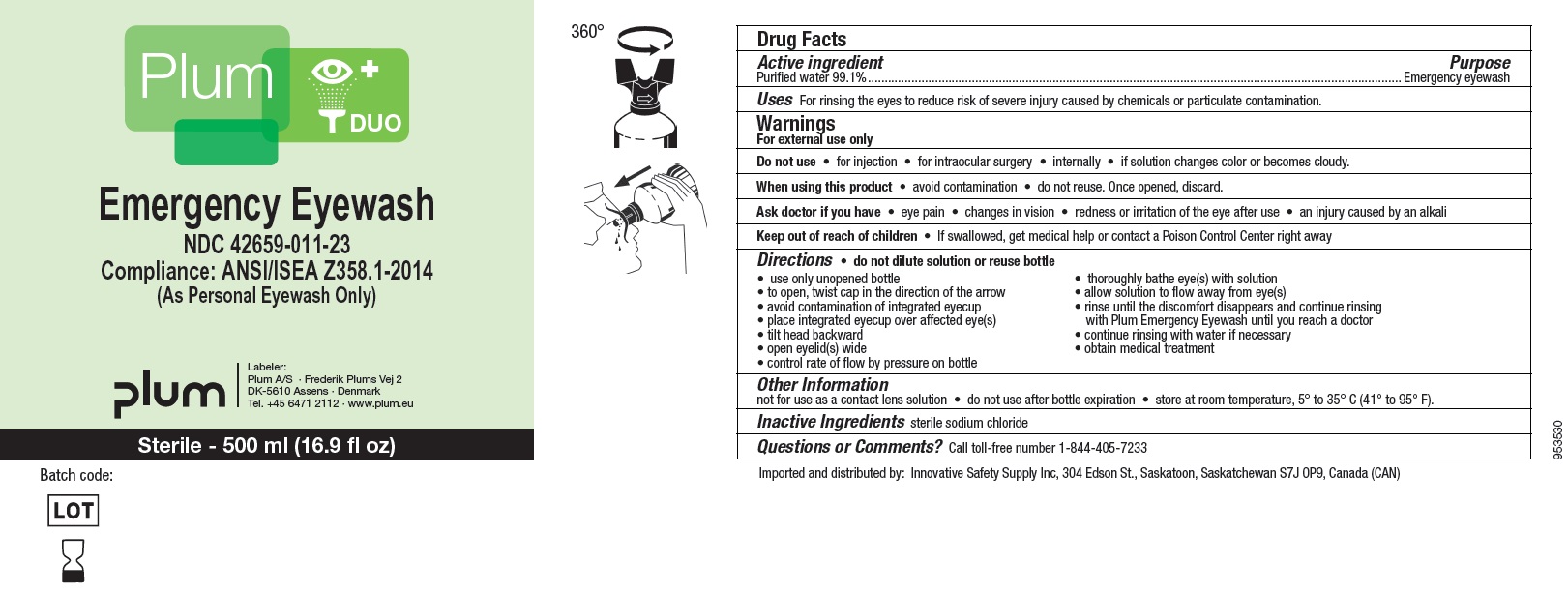
NDC : 42659-011-23
Plum DUO
Emergency Eyewash
Compliance: ANSI/ISEA Z358.1-2014
(As Personal Eyewash Only)
plum
Labeler: Plum A/S - Frederik plums Vej 2
DK - 5610 Assens - Denmark
Tel. +45 6471 2112
www.plum.eu
Sterile - 500 ml (16.9 fl oz)
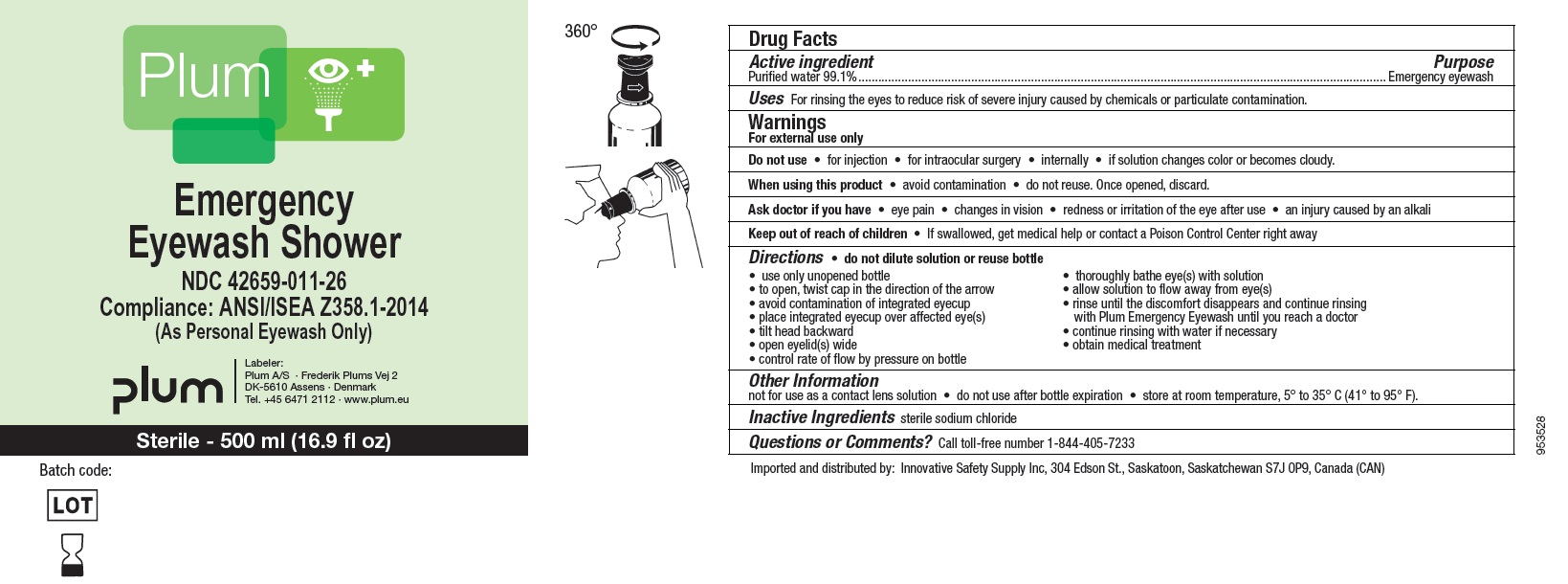
NDC : 42659-011-26
Plum
Emergency Eyewash Shower
Compliance: ANSI/ISEA Z358.1-2014
(As Personal Eyewash Only)
plum
Labeler: Plum A/S - Frederik plums Vej 2
DK - 5610 Assens - Denmark
Tel. +45 6471 2112
www.plum.eu
Sterile - 500 ml (16.9 fl oz)
-
INGREDIENTS AND APPEARANCE
PLUM EMERGENCY EYEWASH/EYEWASH DUO/EYEWASH SHOWER ISS USA
water liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 42659-011 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Water (UNII: 059QF0KO0R) (Water - UNII:059QF0KO0R) Water 99.1 mL in 100 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42659-011-20 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 2 NDC: 42659-011-21 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 3 NDC: 42659-011-22 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 4 NDC: 42659-011-23 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 5 NDC: 42659-011-24 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 6 NDC: 42659-011-26 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 7 NDC: 42659-011-27 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 06/17/2016 Labeler - Plum A/S (305299489) Establishment Name Address ID/FEI Business Operations Plum A/S 305299489 label(42659-011) Establishment Name Address ID/FEI Business Operations Holopack Verpackungstechnik GmbH 343390324 manufacture(42659-011)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.