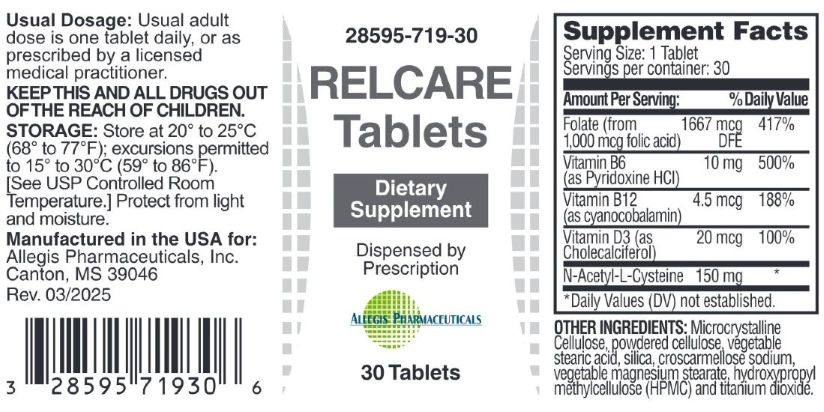
Prenate Max TabletsPRENATAL/POSTNATAL Dietary Supplement
Prenate Max by
Drug Labeling and Warnings
Prenate Max by is a Other medication manufactured, distributed, or labeled by Allegis Pharmaceuticals, LLC, Formulation Tech Incorporated. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PRENATE MAX- beta-crotene, ascorbic acid, cholecalciferol, dl-alpha tocopherol acetate, pyridoxine hci, biotin, folic acid and l-5 mthf ca salt, cyanocobalamin, calcium carbonate, megnesium oxide, ferrous fumarate, postassium iodide, thiamin hci tablet
Allegis Pharmaceuticals, LLC
----------
Prenate Max Tablets
PRENATAL/POSTNATAL
Dietary Supplement
|
Serving Size: 1 Tablet Servings Per Container: 30 | ||
|
Each Tablet Contains: |
%DV |
|
|
Vitamin A (as beta-carotene) |
300 mcg RAE |
66% |
|
Vitamin C (as ascorbic acid) |
125 mg |
140% |
|
Vitamin D3 (as cholecalciferol) |
12.5 mcg |
30% |
|
Vitamin E (as dl-alpha-tocopheryl acetate) |
4.5 mg |
33% |
|
Vitamin B6 (as pyridoxine HCl) |
20 mg |
1117% |
|
Folate (From 588 mcg folic acid and 442 mcg L-5 MTHF ca salt) |
1750 mcg DFE |
437.5% |
|
Vitamin B12 (as cyanocobalamine) |
0.03 mg |
650% |
|
Biotin |
30 mg |
10% |
|
Calcium (from calcium carbonate) |
100 mg |
7.5% |
|
Iron (as ferrous fumarate) |
15 mg |
83% |
|
Iodine (as potassium iodide) |
150 mcg |
100% |
|
Magnesium (as magnesium oxide) |
20 mg |
5% |
|
Thiamin (as thiamin HCI) 1.4 mg 100% |
||
** Daily Values (DV) not established.
OTHER INGREDIENTS: Microcrystalline cellulose, powdered cellulose, silica, vegetable steric acid, croscarmellose sodium, vegetable magnesium stearate, coating (hypromellose, hydroxypropyl cellulose, titanium dioxide, polyehylene glycol).
Description
Prenate Max Tablets is a prescription dietary supplement for use throughout pregnancy, during the postnatal period for both lactating and non-lactating mothers, and throughout the childbearing years.
Prenate Max Tablets may be useful in improving the nutritional status of women prior to conception.
CONTRADINDICATIONS
Prenate Max Tablets are contraindicted in patients with a known hypersensitivity to any of the contained ingredients. Do not take this product if you are presently taking mineral oil, unless directed by a licensed medical practitioner.
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. In the case of accidental overdose, call a doctor or poison control center immediately.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
You may report side effects by calling Allegis Pharmaceuticals, Inc at 1-866-633-9033 or the FDA by calling 1-800-FDA-1088.
DOSAGE AND ADMINISTRATION
Before, during and/or after pregnancy, one tablet daily with food or as directed by a physician.
PRECAUTION:
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. Prenate Max Tablets should only be used under the direction and supervision of a licensed medical practioner.
For use under the supervision of a licensed medical practitioner. Dispense in a tight, light resistant container as defined in the USP/NF in a child-resistant closure.
How Supplied
How Supplied
Prenate Max Talets are available as white tablets with "225" embossed and are available in 30 count bottles (28595-727-30*).
Manufactured in the USA for:
Allegis Pharmaceuticals, Inc.
Canton, MS 39046
Rev 04/2025
Statements
These statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease.
This product is not an Orange Book Product.
Dispense by Prescrition**
*Allegis Pharmaceuticals, Inc. does not represent these product codes to be National Drug Codes (NDC). Product codes are formated according to standard industry practice, to meet the formatting requirements by pedigree reporting and supply-chain control including pharmacies.
** This product is a prescription-folate with or without other dietary ingredients that - due to increased folate levels increased risk associated with masking of B12 deficiency (pernicious anemia) requires administratin under the care of a licensed medical practitioner (61 FR 8760).1-3 The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product only by prescription. This is not an Orange Book product. This product may be administered only under a physician's supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The ingredients, indication or claims of this product are not to be construed to be a drug claims.
1. Federal Register Notice of August 2, 1973 (38 FR 20750)
2. Federal Register Notice of October 17, 1980 (45 FR 69043, 69044)
3. Federal Register Notice of March 5, 1996 (61 FR 8760)
| PRENATE MAX
beta-crotene, ascorbic acid, cholecalciferol, dl-alpha tocopherol acetate, pyridoxine hci, biotin, folic acid and l-5 mthf ca salt, cyanocobalamin, calcium carbonate, megnesium oxide, ferrous fumarate, postassium iodide, thiamin hci tablet |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| shape | ||
| imprint | ||
| size (solid drugs) | 19 mm | |
| scoring | 1 | |
| Labeler - Allegis Pharmaceuticals, LLC (792272861) |