CETIRIZINE HYDROCHLORIDE tablet, chewable
Cetirizine Hydrochloride by
Drug Labeling and Warnings
Cetirizine Hydrochloride by is a Otc medication manufactured, distributed, or labeled by Novel Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
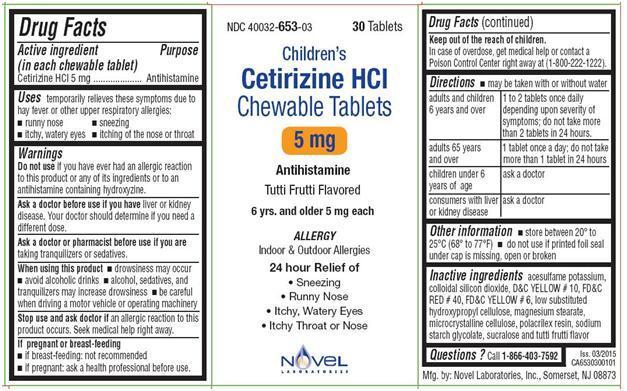
- Active Ingredient in each chewable tablet
- PURPOSE
- Uses
- WARNINGS
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use
- When using this product
- Stop use and ask doctor if
- If pregnant or breast-feeding
- Keep out of the reach of children
-
Directions
- may be taken with or without water
For Cetirizine Hydrochloride Chewable Tablets, 5 mg
adults and children 6 years
1 to 2 tablets once daily depending upon severity of symptoms; do not take
and over
more than 2 tablets in 24 hours.
adults 65 years and over
1 tablet once a day; do not take more than 1 tablet in 24 hours
children under 6 years of
ask a doctor
age
consumers with liver or
ask a doctor
kidney disease
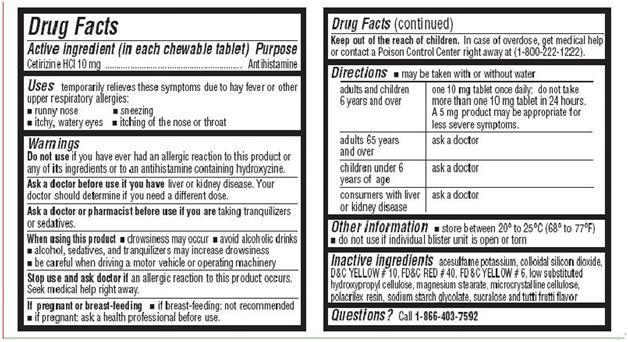
For Cetirizine Hydrochloride Chewable Tablets, 10 mg
adults and children 6
one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A
years and over
5 mg product may be appropriate for less severe symptoms.
adults 65 years and
ask a doctor
over
children under 6 years of age
ask a doctor
consumers with liver
ask a doctor
or kidney disease
- store between 20° to 25°C (68° to 77°F)
- Do not use if individual blister unit is open or torn
- Inactive Ingredients
- QUESTIONS
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 40032-653-03
Children's
Cetirizine Hydrochloride Chewable Tablets
5 mg
ALLERGY
Antihistamine
Indoor & Outdoor Allergies
Tutti-frutti Flavor
6 yrs. & older
30 CHEWABLE TABLETS
Container Label



NDC: 40032-652-31
Children's
Cetirizine Hydrochloride Chewable Tablets
10 mg
ALLERGY
Antihistamine
Indoor & Outdoor Allergies
Tutti-frutti Flavor
6 yrs. & older
Blister Label
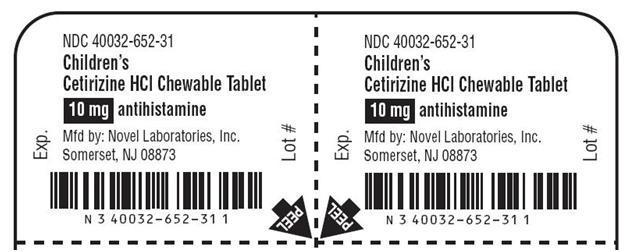
NDC: 40032-652-30

-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 40032-653 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) POLACRILIN (UNII: RCZ785HI7S) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SUCRALOSE (UNII: 96K6UQ3ZD4) MAGNESIUM STEARATE (UNII: 70097M6I30) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color yellow Score no score Shape ROUND Size 7mm Flavor TUTTI FRUTTI Imprint Code n;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 40032-653-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/08/2016 2 NDC: 40032-653-30 3 in 1 CARTON 03/08/2016 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 40032-653-03 1 in 1 CARTON 03/08/2016 3 30 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206793 03/08/2016 CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 40032-652 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) POLACRILIN (UNII: RCZ785HI7S) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SUCRALOSE (UNII: 96K6UQ3ZD4) MAGNESIUM STEARATE (UNII: 70097M6I30) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color yellow Score no score Shape ROUND Size 10mm Flavor TUTTI FRUTTI Imprint Code n;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 40032-652-30 3 in 1 CARTON 03/08/2016 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 40032-652-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/08/2016 3 NDC: 40032-652-03 1 in 1 CARTON 03/08/2016 3 30 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206793 03/08/2016 Labeler - Novel Laboratories, Inc. (793518643) Registrant - Novel Laboratories, Inc. (793518643) Establishment Name Address ID/FEI Business Operations Novel Laboratories, Inc. 793518643 analysis(40032-653, 40032-652) , manufacture(40032-653, 40032-652)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.