LUMAKRAS- sotorasib tablet, coated
LUMAKRAS by
Drug Labeling and Warnings
LUMAKRAS by is a Prescription medication manufactured, distributed, or labeled by Amgen Inc, Amgen, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LUMAKRAS safely and effectively. See full prescribing information for LUMAKRAS.
LUMAKRAS® (sotorasib) tablets, for oral use
Initial U.S. Approval: 2021RECENT MAJOR CHANGES
INDICATIONS AND USAGE
LUMAKRAS is an inhibitor of the RAS GTPase family indicated for:
KRAS G12C-mutated Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC)
- As a single agent, for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who have received at least one prior systemic therapy. (1.1)
This indication is approved under accelerated approval based on overall response rate (ORR) and duration of response (DOR). Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). (1.1)
KRAS G12C-mutated Metastatic Colorectal Cancer (mCRC)
In combination with panitumumab, for the treatment of adult patients with KRAS G12C-mutated mCRC as determined by an FDA approved-test, who have received prior fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy. (1.2)
DOSAGE AND ADMINISTRATION
Recommended dosage as a single agent for NSCLC and in combination with panitumumab for mCRC:
DOSAGE FORMS AND STRENGTHS
Tablets: 320 mg, 240 mg, 120 mg. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Hepatotoxicity: Monitor liver function tests every 3 weeks for the first 3 months of treatment then once monthly as clinically indicated. Consider administering systemic corticosteroids and withhold, reduce the dose, or permanently discontinue LUMAKRAS based on the severity. (2.3, 5.1)
- Interstitial Lung Disease (ILD)/Pneumonitis: Monitor for new or worsening pulmonary symptoms. Immediately withhold LUMAKRAS for suspected ILD/pneumonitis and permanently discontinue if no other potential causes of ILD/pneumonitis are identified. (2.3, 5.2)
ADVERSE REACTIONS
- Single agent in NSCLC: The most common adverse reactions (≥ 20%) were diarrhea, musculoskeletal pain, nausea, fatigue, hepatotoxicity, and cough. The most common laboratory abnormalities (≥ 25%) were decreased lymphocytes, decreased hemoglobin, increased aspartate aminotransferase, increased alanine aminotransferase, decreased calcium, increased alkaline phosphatase, increased urine protein, and decreased sodium. (6.1)
- In combination with panitumumab in CRC: The most common adverse reactions (≥ 20%) in clinical trials of LUMAKRAS in combination with panitumumab are rash, dry skin, diarrhea, stomatitis, fatigue and musculoskeletal pain. The most common Grade 3 or 4 laboratory abnormalities in ≥ 2 patients (4.3%) were decreased magnesium, decreased potassium, decreased corrected calcium, and increased potassium. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amgen Inc. at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Acid-Reducing Agents: Avoid coadministration with proton pump inhibitors (PPIs) and H2 receptor antagonists. If an acid-reducing agent cannot be avoided, administer LUMAKRAS 4 hours before or 10 hours after a local antacid. (2.4, 7.1)
- Strong CYP3A4 Inducers: Avoid coadministration with strong CYP3A4 inducers. (7.1)
- CYP3A4 Substrates: Avoid coadministration with CYP3A4 substrates for which minimal concentration changes may lead to therapeutic failures of the substrate. If coadministration cannot be avoided, adjust the substrate dosage in accordance to its Prescribing Information. (7.2)
- P-gp substrates: Avoid coadministration with P-gp substrates for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the substrate dosage in accordance to its Prescribing Information. (7.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 KRAS G12C-mutated Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC)
1.2 KRAS G12C-mutated Metastatic Colorectal Cancer (mCRC)
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dosage and Administration
2.3 Dosage Modifications for Adverse Reactions
2.4 Coadministration of LUMAKRAS with Acid-Reducing Agents
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity
5.2 Interstitial Lung Disease (ILD)/Pneumonitis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on LUMAKRAS
7.2 Effects of LUMAKRAS on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 KRAS G12C-mutated Locally Advanced or Metastatic Non-Small Cell Lung Cancer
14.2 KRAS G12C-mutated Metastatic Colorectal Cancer
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 KRAS G12C-mutated Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC)
LUMAKRAS as a single agent is indicated for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), as determined by an FDA-approved test [see Dosage and Administration (2.1)], who have received at least one prior systemic therapy.
This indication is approved under accelerated approval based on overall response rate (ORR) and duration of response (DOR) [see Clinical Studies (14.1)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
1.2 KRAS G12C-mutated Metastatic Colorectal Cancer (mCRC)
LUMAKRAS, in combination with panitumumab, is indicated for the treatment of adult patients with KRAS G12C-mutated metastatic colorectal cancer (mCRC), as determined by an FDA-approved test, who have received prior fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy [see Dosage and Administration (2.1) and Clinical Studies (14.2)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
KRAS G12C-mutated Locally Advanced or Metastatic NSCLC
Select patients for treatment of locally advanced or metastatic NSCLC with LUMAKRAS based on the presence of KRAS G12C mutation in tumor or plasma specimens. If no mutation is detected in a plasma specimen, test tumor tissue [see Clinical Studies (14.1)].
KRAS G12C-mutated mCRC
Select patients for treatment of mCRC based on the presence of KRAS G12C mutation in tumor specimens [see Clinical Studies (14.2)].
Information on FDA-approved tests for the detection of KRAS G12C mutations is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage and Administration
LUMAKRAS as a Single Agent for KRAS G12C-mutated Locally Advanced or Metastatic NSCLC
The recommended dosage of LUMAKRAS is 960 mg (three 320 mg tablets or four 240 mg tablets or eight 120 mg tablets) orally once daily until disease progression or unacceptable toxicity.
LUMAKRAS in Combination with Panitumumab for KRAS G12C-mutated mCRC
The recommended dosage of LUMAKRAS is 960 mg (three 320 mg tablets or four 240 mg tablets or eight 120 mg tablets) orally once daily in combination with panitumumab until disease progression or unacceptable toxicity. Administer the first dose of LUMAKRAS prior to first panitumumab infusion.
Refer to the panitumumab full prescribing information for recommended panitumumab dosage information.
Take the daily dose of LUMAKRAS at the same time each day with or without food [see Clinical Pharmacology (12.3)]. Swallow tablets whole. Do not chew, crush or split tablets. If a dose of LUMAKRAS is missed by more than 6 hours, take the next dose as prescribed the next day. Do not take 2 doses at the same time to make up for the missed dose.
If vomiting occurs after taking LUMAKRAS, do not take an additional dose. Take the next dose as prescribed the next day.
Administration to Patients Who Have Difficulty Swallowing Solids
Disperse tablets in 120 mL (4 ounces) of non-carbonated, room-temperature water without crushing. No other liquids should be used. Stir or swirl the cup for approximately 3 minutes until tablets are dispersed into small pieces (the tablets will not completely dissolve) and drink immediately or within 2 hours. The appearance of the mixture may range from pale yellow to bright yellow. Swallow the tablet dispersion. Do not chew pieces of the tablet. Rinse the container with an additional 120 mL (4 ounces) of water and drink. If the mixture is not consumed immediately, stir the mixture again to ensure that tablets are dispersed.
2.3 Dosage Modifications for Adverse Reactions
LUMAKRAS dose reduction levels are summarized in Table 1.
If adverse reactions occur, a maximum of two dose reductions are permitted. Discontinue LUMAKRAS if patients are unable to tolerate the minimum dose of 240 mg once daily.
When LUMAKRAS is administered in combination with panitumumab, and LUMAKRAS is temporarily withheld or permanently discontinued, temporarily withhold or permanently discontinue panitumumab, respectively [see Clinical Studies (14.2)]. Refer to the full prescribing information of panitumumab for dose modifications for adverse reactions associated with the use of panitumumab.
Treatment with LUMAKRAS as a single agent may be continued if panitumumab is permanently discontinued [see Clinical Pharmacology (12.1), Clinical Studies (14.2)].
Refer to Table 2 for dose modification guidelines and management of adverse reactions associated with the use of LUMAKRAS as a single agent or as combination therapy with panitumumab.
Table 1. Recommended LUMAKRAS Dose Reduction Levels for Adverse Reactions Dose Reduction Level Dose First dose reduction 480 mg (two 240 mg or four 120 mg tablets) once daily Second dose reduction 240 mg (one 240 mg or two 120 mg tablets) once daily Table 2. Recommended LUMAKRAS Dosage Modifications for Adverse Reactions Adverse Reaction Severity* Dosage Modification† ALT = alanine aminotransferase; AST = aspartate aminotransferase; ULN = upper limit of normal - * Grading defined by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0.
- † When LUMAKRAS is administered in combination with panitumumab, withhold or permanently discontinue treatment with panitumumab when withholding or permanently discontinuing treatment with LUMAKRAS.
Hepatotoxicity [see Warnings and Precautions (5.1)] AST or ALT > 3 × and up to 5 × ULN (or > 3 × and up to 5 × baseline if baseline abnormal) with symptoms
or
AST or ALT > 5 × ULN (or > 5 × baseline if baseline abnormal)- Withhold LUMAKRAS until recovery to ≤ 3 × ULN or to ≤ 3 × baseline if baseline abnormal.
- Resume LUMAKRAS at the next lower dose level.
AST or ALT > 3 × ULN with total bilirubin > 2 × ULN - Permanently discontinue LUMAKRAS if no alternative cause is identified.
- If alternative cause is identified, do not resume LUMAKRAS until AST/ALT/bilirubin return to baseline.
Interstitial Lung Disease (ILD)/ pneumonitis [see Warnings and Precautions (5.2)] Any Grade - Withhold LUMAKRAS if ILD/pneumonitis is suspected.
- Permanently discontinue LUMAKRAS if ILD/pneumonitis is confirmed.
Nausea or vomiting despite appropriate supportive care (including anti-emetic therapy)
[see Adverse Reactions (6.1)]Grade 3 to 4 - Withhold LUMAKRAS until recovery to ≤ Grade 1 or baseline.
- Resume LUMAKRAS at the next lower dose level.
Diarrhea despite appropriate supportive care (including anti-diarrheal therapy)
[see Adverse Reactions (6.1)]Grade 3 to 4 - Withhold LUMAKRAS until recovery to ≤ Grade 1 or baseline.
- Resume LUMAKRAS at the next lower dose level.
Other adverse reactions
[see Adverse Reactions (6.1)]Grade 3 to 4 - Withhold LUMAKRAS until recovery to ≤ Grade 1 or baseline.
- Resume LUMAKRAS at the next lower dose level.
2.4 Coadministration of LUMAKRAS with Acid-Reducing Agents
Avoid coadministration of proton pump inhibitors (PPIs) and H2 receptor antagonists with LUMAKRAS. If treatment with an acid-reducing agent cannot be avoided, take LUMAKRAS 4 hours before or 10 hours after administration of a local antacid [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
Tablets: 320 mg, beige, oval-shaped, immediate release, film-coated, debossed with "AMG" on one side and "320" on the opposite side.
Tablets: 240 mg, yellow, oval-shaped, immediate release, film-coated, debossed with "AMG" on one side and "240" on the opposite side.
Tablets: 120 mg, yellow, oblong-shaped, immediate release, film-coated, debossed with "AMG" on one side and "120" on the opposite side.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity
LUMAKRAS can cause hepatotoxicity and increased alanine aminotransferase (ALT) or increased aspartate aminotransferase (AST) which may lead to drug-induced liver injury and hepatitis.
In the pooled safety population of patients with NSCLC who received single agent LUMAKRAS 960 mg [see Adverse Reactions (6.1)], hepatotoxicity occurred in 27% of patients, of which 16% were Grade ≥ 3. Among patients with hepatotoxicity who required dosage modifications, 64% required treatment with corticosteroids.
In this pooled safety population of patients with NSCLC who received single agent LUMAKRAS 960 mg, 17% of patients who received LUMAKRAS had increased ALT/increased AST; of which 9% were Grade ≥ 3. The median time to first onset of increased ALT/AST was 6.3 weeks (range: 0.4 to 42). Increased ALT/AST leading to dose interruption or reduction occurred in 9% of patients treated with LUMAKRAS. LUMAKRAS was permanently discontinued due to increased ALT/AST in 2.7% of patients. Drug-induced liver injury occurred in 1.6% (all grades) including 1.3% (Grade ≥ 3).
In this pooled safety population of patients with NSCLC who received single agent LUMAKRAS 960 mg, a total of 40% patients with recent (≤ 3 months) immunotherapy prior to starting LUMAKRAS had an event of hepatotoxicity. An event of hepatotoxicity was observed in 18% of patients who started LUMAKRAS more than 3 months after last dose of immunotherapy and in 17% of those who never received immunotherapy. Regardless of time from prior immunotherapy, 94% of hepatotoxicity events improved or resolved with dosage modification of LUMAKRAS, with or without corticosteroid treatment.
In the pooled safety population of patients with CRC who received LUMAKRAS 960 mg in combination with panitumumab [see Adverse Reactions (6.1)], hepatotoxicity occurred in 15% of patients, of which 4.8% were Grade 3. A total of 7% of patients who received LUMAKRAS had increased ALT or increased AST, of which 0.8% were Grade 3. The median time to first onset of increased ALT or increased AST was 10 weeks (range: 2 to 22). Increased ALT or increased AST leading to dose interruption occurred in 2.4% of patients. A total of 3.2% of patients who received LUMAKRAS had hyperbilirubinemia, of which 2.4% were Grade 3. The median time to first onset of hyperbilirubinemia was 12 weeks (range: 0, 29). Hyperbilirubinemia leading to dose interruption occurred in 1.6% of patients. Among patients with hepatotoxicity, 21% received corticosteroids.
Monitor liver function tests (ALT, AST, alkaline phosphatase and total bilirubin) prior to the start of LUMAKRAS, every 3 weeks for the first 3 months of treatment, then once a month or as clinically indicated, with more frequent testing in patients who develop transaminase and/or bilirubin elevations. Withhold, reduce the dose or permanently discontinue LUMAKRAS based on severity of the adverse reaction [see Dosage and Administration (2.3) and Adverse Reactions (6.1)]. Consider administering systemic corticosteroids for the management of hepatotoxicity.
5.2 Interstitial Lung Disease (ILD)/Pneumonitis
LUMAKRAS can cause ILD/pneumonitis that can be fatal.
In the pooled safety population of patients with NSCLC who received single agent LUMAKRAS 960 mg [see Adverse Reactions (6.1)], ILD/pneumonitis occurred in 2.2% of patients, of which 1.1% were Grade ≥ 3, and 1 case was fatal. The median time to first onset for ILD/pneumonitis was 8.6 weeks (range: 2.1 to 36.7 weeks). LUMAKRAS was permanently discontinued due to ILD/pneumonitis in 1.3% of LUMAKRAS-treated patients. Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis (e.g., dyspnea, cough, fever). Immediately withhold LUMAKRAS in patients with suspected ILD/pneumonitis and permanently discontinue LUMAKRAS if no other potential causes of ILD/pneumonitis are identified [see Dosage and Administration (2.3) and Adverse Reactions (6.1)].
In the pooled safety population of patients with CRC who received LUMAKRAS 960 mg in combination with panitumumab, 1 patient experienced a Grade 1 event of ILD/pneumonitis [see Adverse Reactions (6.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Hepatotoxicity [see Warnings and Precautions (5.1)]
- Interstitial Lung Disease (ILD)/Pneumonitis [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the WARNINGS AND PRECAUTIONS reflect exposure to LUMAKRAS as a single agent at 960 mg orally once daily until disease progression or unacceptable toxicity in 549 patients with NSCLC with KRAS G12C mutation in the following trials: CodeBreaK 200 (NCT04303780), CodeBreaK 100 (NCT03600883), CodeBreaK 101 (NCT04185883) and CodeBreaK 105 (NCT04380753). Among these 549 patients who received LUMAKRAS, 44% were exposed for 6 months or longer and 21% were exposed for greater than one year.
The pooled safety population described in WARNINGS AND PRECAUTIONS also reflects exposure to LUMAKRAS 960 mg once daily in combination with panitumumab in 126 patients who received LUMAKRAS in combination with panitumumab for mCRC in CodeBreaK 300 (NCT05198934) and CodeBreaK 101 (NCT04185883). Among the 126 patients who received LUMAKRAS 960 mg in combination with panitumumab, 40% were exposed for 6 months or longer and 10% were exposed for greater than one year.
Metastatic Non-Small Cell Lung Cancer
The safety of LUMAKRAS was evaluated in a subset of patients with KRAS G12C-mutated locally advanced or metastatic NSCLC in CodeBreaK 100 [see Clinical Studies (14.1)]. Patients received LUMAKRAS 960 mg orally once daily until disease progression or unacceptable toxicity (n = 204). Among patients who received LUMAKRAS, 39% were exposed for 6 months or longer and 3% were exposed for greater than one year.
The median age of patients who received LUMAKRAS was 66 years (range: 37 to 86); 55% female; 80% White, 15% Asian, and 3% Black.
Serious adverse reactions occurred in 50% of patients treated with LUMAKRAS. Serious adverse reactions in ≥ 2% of patients were pneumonia (8%), hepatotoxicity (3.4%), and diarrhea (2%). Fatal adverse reactions occurred in 3.4% of patients who received LUMAKRAS due to respiratory failure (0.8%), pneumonitis (0.4%), cardiac arrest (0.4%), cardiac failure (0.4%), gastric ulcer (0.4%), and pneumonia (0.4%).
Permanent discontinuation of LUMAKRAS due to an adverse reaction occurred in 9% of patients. Adverse reactions resulting in permanent discontinuation of LUMAKRAS in ≥ 2% of patients included hepatotoxicity (4.9%).
Dosage interruptions of LUMAKRAS due to an adverse reaction occurred in 34% of patients. Adverse reactions which required dosage interruption in ≥ 2% of patients were hepatotoxicity (11%), diarrhea (8%), musculoskeletal pain (3.9%), nausea (2.9%), and pneumonia (2.5%).
Dose reductions of LUMAKRAS due to an adverse reaction occurred in 5% of patients. Adverse reactions which required dose reductions in ≥ 2% of patients included increased ALT (2.9%) and increased AST (2.5%).
The most common adverse reactions (≥ 20%) were diarrhea, musculoskeletal pain, nausea, fatigue, hepatotoxicity, and cough. The most common laboratory abnormalities (≥ 25%) were decreased lymphocytes, decreased hemoglobin, increased aspartate aminotransferase, increased alanine aminotransferase, decreased calcium, increased alkaline phosphatase, increased urine protein, and decreased sodium.
Table 3 summarizes the common adverse reactions observed in CodeBreaK 100.
Table 3. Adverse Reactions (≥ 10%) of Patients with KRAS G12C-Mutated NSCLC who Received LUMAKRAS in CodeBreaK 100* Adverse Reaction LUMAKRAS
N = 204All Grades (%) Grades 3 to 4
(%)- * Grading defined by NCI CTCAE version 5.0.
- † Abdominal pain includes abdominal pain, abdominal pain upper, and abdominal pain lower.
- ‡ Hepatotoxicity includes alanine aminotransferase increased, aspartate aminotransferase increased, blood bilirubin increased, drug-induced liver injury, hepatitis, hepatotoxicity, liver function test increased, and transaminases increased.
- § Cough includes cough, productive cough, and upper-airway cough syndrome.
- ¶ Dyspnea includes dyspnea and dyspnea exertional.
- # Musculoskeletal pain includes back pain, bone pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal pain, myalgia, neck pain, non-cardiac chest pain, and pain in extremity.
- Þ Fatigue includes fatigue and asthenia.
- ß Edema includes generalized edema, localized edema, edema, edema peripheral, periorbital edema, and testicular edema.
- à Pneumonia includes pneumonia, pneumonia aspiration, pneumonia bacterial, and pneumonia staphylococcal.
- è Rash includes dermatitis, dermatitis acneiform, rash, rash-maculopapular, and rash pustular.
Gastrointestinal disorders Diarrhea 42 5 Nausea 26 1 Vomiting 17 1.5 Constipation 16 0.5 Abdominal pain† 15 1.0 Hepatobiliary disorders Hepatotoxicity‡ 25 12 Respiratory, thoracic, and mediastinal disorders Cough§ 20 1.5 Dyspnea¶ 16 2.9 Musculoskeletal and connective tissue disorders Musculoskeletal pain# 35 8 Arthralgia 12 1.0 General disorders and administration site conditions FatigueÞ 26 2.0 Edemaß 15 0 Metabolism and nutrition disorders Decreased appetite 13 1.0 Infections and infestations Pneumoniaà 12 7 Skin and subcutaneous tissue disorders Rashè 12 0 Table 4 summarizes the selected laboratory adverse reactions observed in CodeBreaK 100.
Table 4. Select Laboratory Abnormalities (≥ 20%) that Worsened from Baseline in Patients with KRAS G12C-Mutated NSCLC who Received LUMAKRAS in CodeBreaK 100 Laboratory Abnormalities LUMAKRAS
N = 204*Grades 1 to 4
(%)Grades 3 to 4
(%)- * N = number of patients who had at least one on-study assessment for the parameter of interest.
Chemistry Increased aspartate aminotransferase 39 9 Increased alanine aminotransferase 38 11 Decreased calcium 35 0 Increased alkaline phosphatase 33 2.5 Increased urine protein 29 3.9 Decreased sodium 28 1.0 Decreased albumin 22 0.5 Hematology Decreased lymphocytes 48 2 Decreased hemoglobin 43 0.5 Increased activated partial thromboplastin time 23 1.5 Metastatic Colorectal Cancer
The safety of LUMAKRAS in combination with panitumumab was evaluated in the CodeBreaK 300 study [see Clinical Studies (14.2)]. Patients with KRAS G12C-mutated mCRC received LUMAKRAS 960 mg orally once daily in combination with panitumumab 6 mg/kg intravenously (IV) once every 2 weeks (N = 47), LUMAKRAS 240 mg orally once daily in combination with panitumumab 6 mg/kg IV once every 2 weeks (N = 50), or the investigator's choice of standard of care (SOC) trifluridine/ tipiracil or regorafenib (N = 50). Among patients who received LUMAKRAS 960 mg orally once daily in combination with panitumumab, 36% were exposed to LUMAKRAS for greater than 6 months and 6% were exposed for greater than 12 months.
The median age of patients who received LUMAKRAS 960 mg in combination with panitumumab arm was 63 years (range: 37-79 years); 38% were age 65 years or older; 49% were female; 79% were White and 13% were Asian.
Serious adverse reactions occurred in 26% of patients receiving LUMAKRAS 960 mg in combination with panitumumab. Serious adverse reactions in ≥ 2 patients receiving LUMAKRAS 960 mg in combination with panitumumab were sepsis (6%) and intestinal obstruction (4.3%). Fatal adverse reactions occurred in 2 patients (4.3%) receiving LUMAKRAS 960 mg in combination with panitumumab, consisting of cardiac arrest and sepsis (1 patient each).
Permanent discontinuation of LUMAKRAS due to an adverse reaction occurred in 1 patient for decreased corrected calcium.
Dosage interruptions of LUMAKRAS due to an adverse reaction occurred in 26% of patients. Adverse reactions which required dosage interruption in ≥ 2 patients were rash, hepatotoxicity and intestinal obstruction.
A dose reduction of LUMAKRAS due to an adverse reaction occurred in 1 patient for nausea.
The most common adverse reactions (≥ 20%) in patients receiving LUMAKRAS 960 mg in combination with panitumumab were rash, dry skin, diarrhea, stomatitis, fatigue and musculoskeletal pain.
The most common Grade 3-4 laboratory abnormalities in ≥ 2 patients (4.3%) were decreased magnesium, decreased potassium, decreased corrected calcium, and increased potassium.
Table 5 and Table 6 summarize the adverse reactions and laboratory abnormalities, respectively, identified in CodeBreaK 300.
Table 5. Adverse Reactions (≥ 10%) in Patients with KRAS G12C-Mutated mCRC who Received LUMAKRAS 960 mg in Combination with Panitumumab in CodeBreaK 300 Adverse Reaction LUMAKRAS 960 mg in combination with panitumumab
N = 47Trifluridine/tipiracil or regorafenib
N = 50All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * Rash includes dermatitis acneiform, dermatosis, drug eruption, eczema, erythema, hand dermatitis, rash, rash erythematous, rash maculo-papular, rash papular, rash pruritic, rash pustular, and skin toxicity.
- † Dry skin includes dry skin, xerosis, and xeroderma.
- ‡ Nail disorders includes nail avulsion, nail cuticle fissure, nail disorder, nail toxicity, and paronychia.
- § Diarrhea includes diarrhea, gastroenteritis, and diarrhea hemorrhagic.
- ¶ Stomatitis includes mucosal inflammation, stomatitis, mouth ulceration, angular cheilitis, and cheilitis.
- # Abdominal pain includes abdominal pain, abdominal pain upper, abdominal pain lower, abdominal discomfort, and hepatic pain.
- Þ Fatigue includes asthenia and fatigue.
- ß Musculoskeletal pain includes arthralgia, back pain, myalgia, musculoskeletal chest pain, bone pain, and pain in extremity.
- à Hemorrhage includes epistaxis, gastrointestinal hemorrhage, vaginal hemorrhage, rectal hemorrhage, hematochezia, hemorrhage, hemorrhage urinary tract, hematospermia, and hematuria.
- è Conjunctivitis includes conjunctival hyperemia, conjunctivitis, and conjunctivitis allergic.
Skin and subcutaneous tissue disorders Rash* 87 26 8 2 Dry skin† 28 0 2 0 Pruritis 17 0 4 0 Nail Disorder‡ 17 0 0 0 Skin fissure 13 0 0 0 Palmar-plantar erythrodysesthesia syndrome 13 0 10 4 Gastrointestinal disorders Diarrhea§ 28 6 26 0 Stomatitis¶ 26 0 14 0 Nausea 17 2.1 36 4 Constipation 15 2.1 10 0 Abdominal pain# 15 0 18 2 Vomiting 13 2.1 10 2 General disorders FatigueÞ 21 0 34 2 Musculoskeletal and connective tissue disorders Musculoskeletal painß 21 2.1 14 2 Hematological disorders Hemorrhageà 13 2.1 2 0 Eye disorders Conjunctivitisè 11 0 2 0 Table 6. Select Laboratory Abnormalities (≥ 20%) that Worsened from Baseline in Patients with KRAS G12C-Mutated mCRC who Received LUMAKRAS 960 mg in combination with panitumumab in CodeBreaK 300* Laboratory Abnormalities LUMAKRAS 960 mg in combination with panitumumab Trifluridine/tipiracil or Regorafenib All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- * The denominator used to calculate the rate varied from 44 to 46 in the LUMAKRAS + panitumumab arm and 18 to 50 in the trifluridine/tipiracil or regorafenib arm based on the number of patients with a baseline value and at least one post-treatment value.
Chemistry Magnesium decreased 76 24 8 0 Calcium (corrected) decreased 74 4.3 46 0 Aspartate aminotransferase increased 39 0 22 2 Alkaline Phosphatase increased 33 2.2 33 0 Creatine kinase increased 30 2.3 7 0 Alanine Aminotransferase increased 28 0 16 2 Potassium decreased 26 7 12 0 Albumin decreased 26 2.2 22 0 Urine protein increased 23 0 22 6 Potassium increased 22 4.3 6 0 Glucose decreased 22 0 2 0 Hematology Hemoglobin decreased 30 0 58 6 Lymphocytes decreased 26 2.2 56 8 White blood cells decreased 24 0 48 14 -
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on LUMAKRAS
Acid-Reducing Agents
The solubility of sotorasib is pH-dependent. Coadministration of LUMAKRAS with gastric acid-reducing agents decreased sotorasib concentrations [see Clinical Pharmacology (12.3)], which may reduce the efficacy of sotorasib. Avoid coadministration of LUMAKRAS with proton pump inhibitors (PPIs), H2 receptor antagonists, and locally acting antacids. If coadministration with an acid-reducing agent cannot be avoided, administer LUMAKRAS 4 hours before or 10 hours after administration of a locally acting antacid [see Dosage and Administration (2.4)].
Strong CYP3A4 Inducers
Sotorasib is a CYP3A4 substrate. Coadministration of LUMAKRAS with a strong CYP3A4 inducer decreased sotorasib concentrations [see Clinical Pharmacology (12.3)], which may reduce the efficacy of sotorasib. Avoid coadministration of LUMAKRAS with strong CYP3A4 inducers.
7.2 Effects of LUMAKRAS on Other Drugs
CYP3A4 Substrates
Sotorasib is a CYP3A4 inducer. Coadministration of LUMAKRAS with a CYP3A4 substrate decreased its plasma concentrations [see Clinical Pharmacology (12.3)], which may reduce the efficacy of the substrate. Avoid coadministration of LUMAKRAS with CYP3A4 sensitive substrates, for which minimal concentration changes may lead to therapeutic failures of the substrate. If coadministration cannot be avoided, increase the sensitive CYP3A4 substrate dosage in accordance with its Prescribing Information.
P-glycoprotein (P-gp) Substrates
Sotorasib is a P-gp inhibitor. Coadministration of LUMAKRAS with a P-gp substrate increased its plasma concentrations [see Clinical Pharmacology (12.3)], which may increase the adverse reactions of the substrate. Avoid coadministration of LUMAKRAS with P-gp substrates, for which minimal concentration changes may lead to serious toxicities. If coadministration cannot be avoided, decrease the P-gp substrate dosage in accordance with its Prescribing Information.
Breast Cancer Resistance Protein (BCRP) Substrates
Sotorasib is a BCRP-inhibitor. Coadministration of LUMAKRAS with a BCRP substrate increased its plasma concentrations [see Clinical Pharmacology (12.3)], which may increase the risk of adverse reactions of the substrate. When coadministered with LUMAKRAS, monitor for adverse reactions of the BCRP substrate and decrease the BCRP substrate dosage in accordance with its Prescribing Information.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on LUMAKRAS use in pregnant women. In rat and rabbit embryo-fetal development studies, oral sotorasib did not cause adverse developmental effects or embryo-lethality at exposures up to 4.6 times the human exposure at the 960 mg clinical dose (see Data).
Refer to the Full Prescribing Information of panitumumab for pregnancy risk information and contraception recommendations when LUMAKRAS is administered in combination with panitumumab.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In a rat embryo-fetal development study, once daily oral administration of sotorasib to pregnant rats during the period of organogenesis resulted in maternal toxicity at the 540 mg/kg dose level (approximately 4.6 times the human exposure based on area under the curve (AUC) at the clinical dose of 960 mg). Sotorasib did not cause adverse developmental effects and did not affect embryo-fetal survival at doses up to 540 mg/kg.
In a rabbit embryo-fetal development study, once daily oral administration of sotorasib during the period of organogenesis resulted in lower fetal body weights and a reduction in the number of ossified metacarpals in fetuses at the 100 mg/kg dose level (approximately 2.6 times the human exposure based on AUC at the clinical dose of 960 mg), which was associated with maternal toxicity including decreased body weight gain and food consumption during the dosing phase. Sotorasib did not cause adverse developmental effects and did not affect embryo-fetal survival at doses up to 100 mg/kg.
8.2 Lactation
Risk Summary
There are no data on the presence of sotorasib or its metabolites in human milk, the effects on the breastfed child, or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with LUMAKRAS and for 1 week after the last dose.
Refer to the Full Prescribing Information of panitumumab for lactation recommendations when LUMAKRAS is administered in combination with panitumumab.
8.4 Pediatric Use
The safety and effectiveness of LUMAKRAS have not been established in pediatric patients.
8.5 Geriatric Use
Of the 357 patients with any tumor type who received LUMAKRAS 960 mg orally once daily in CodeBreaK 100, 46% were 65 and over, and 10% were 75 and over. No overall differences in safety or effectiveness were observed between older patients and younger patients treated with LUMAKRAS as a single agent.
In a pooled analysis of 132 patients who received LUMAKRAS 960 mg in combination with panitumumab for KRAS G12C-mutated mCRC, 30% were 65 and over while 9% were 75 and over. No overall differences in safety or efficacy were observed between older patients (≥ 65 years of age) compared to younger patients, treated with LUMAKRAS 960 mg in combination with panitumumab.
8.6 Hepatic Impairment
No dosage modification is recommended in patients with mild to moderate hepatic impairment (Child-Pugh A or B).
The effect of severe hepatic impairment (Child-Pugh C) on the safety of LUMAKRAS is unknown. Monitor for sotorasib adverse reactions in patients with hepatic impairment more frequently since these patients may be at increased risk for adverse reactions including hepatotoxicity [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
Sotorasib is an inhibitor of the RAS GTPase family. The molecular formula is C30H30F2N6O3, and the molecular weight is 560.6 g/mol. The chemical name of sotorasib is 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-(1M)-1-[4-methyl-2-(propan-2-yl)pyridin-3-yl]-4-[(2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl]pyrido[2,3-d]pyrimidin-2(1H)-one.
The chemical structure of sotorasib is shown below:
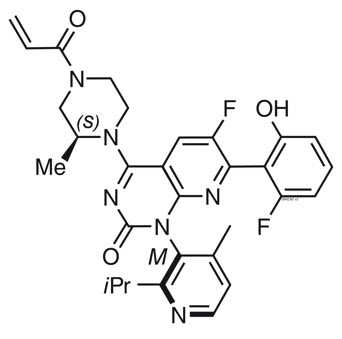
Sotorasib has pKa values of 8.06 and 4.56. The solubility of sotorasib in the aqueous media decreases over the range pH 1.2 to 6.8 from 1.3 mg/mL to 0.03 mg/mL.
LUMAKRAS is supplied as film-coated tablets for oral use containing 320 mg, 240 mg or 120 mg of sotorasib. Inactive ingredients in the tablet core are microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, and magnesium stearate. The film coating material consists of polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, iron oxide yellow and iron oxide red (320 mg tablet only).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sotorasib is an inhibitor of KRAS G12C, a tumor-restricted, mutant-oncogenic form of the RAS GTPase, KRAS. Sotorasib forms an irreversible, covalent bond with the unique cysteine of KRAS G12C, locking the protein in an inactive state that prevents downstream signaling without affecting wild-type KRAS. Sotorasib blocked KRAS signaling, inhibited cell growth, and promoted apoptosis only in KRAS G12C tumor cell lines. Sotorasib inhibited KRAS G12C in vitro and in vivo with minimal detectable off-target activity. In mouse tumor xenograft models, sotorasib-treatment led to tumor regressions and prolonged survival, and was associated with anti-tumor immunity in KRAS G12C models.
In the setting of KRAS G12C-mutant CRC, epidermal growth factor receptor (EGFR) activation has been identified as a mechanism of resistance to KRAS G12C inhibition. In a murine patient-derived colorectal tumor xenograft model, the combination of sotorasib and panitumumab, an EGFR antagonist, had increased antitumor activity compared to either sotorasib or panitumumab alone.
12.2 Pharmacodynamics
Sotorasib exposure-response relationships and the time course of the pharmacodynamic response are unknown.
12.3 Pharmacokinetics
The pharmacokinetics of sotorasib have been characterized in healthy subjects and in patients with KRAS G12C-mutated solid tumors, including NSCLC. Sotorasib exhibited non-linear, time-dependent, pharmacokinetics over the dose range of 180 mg to 960 mg (0.19 to 1 time the approved recommended dosage) once daily with similar systemic exposure (i.e., AUC0-24h and Cmax) across doses at steady state. Sotorasib systemic exposure was comparable between film-coated tablets and film-coated tablets predispersed in water administered under fasted conditions. Sotorasib plasma concentrations reached steady state within 22 days. No accumulation was observed after repeat LUMAKRAS dosages with a mean accumulation ratio of 0.56 (coefficient of variation (CV): 59%).
Absorption
The median time to sotorasib peak plasma concentration is 1 hour.
Effect of Food
When 960 mg LUMAKRAS was administered with a high-fat, high-calorie meal (containing approximately 800 to 1000 calories with 150, 250, and 500 to 600 calories from protein, carbohydrate and fat, respectively) in patients, sotorasib AUC0-24h increased by 25% compared to administration under fasted conditions.
Distribution
The sotorasib mean volume of distribution (Vd) at steady state is 211 L (CV: 135%). In vitro, sotorasib plasma protein binding is 89%.
Elimination
The sotorasib mean terminal elimination half-life is 5 hours (standard deviation (SD): 2). At 960 mg LUMAKRAS once daily, the sotorasib steady state apparent clearance is 26.2 L/hr (CV: 76%).
Specific Populations
No clinically meaningful differences in the pharmacokinetics of sotorasib were observed based on age (28 to 86 years), sex, race (White, Black and Asian), body weight (36.8 to 157.9 kg), line of therapy, ECOG PS (0, 1), mild and moderate renal impairment (eGFR: ≥ 30 mL/min/1.73 m2), or mild hepatic impairment (AST or ALT < 2.5 × ULN or total bilirubin < 1.5 × ULN). The effect of severe renal impairment on sotorasib pharmacokinetics has not been studied.
Hepatic Impairment
The mean AUC of sotorasib decreased by 25% in subjects with moderate hepatic impairment (Child-Pugh B) and increased by 4% in subjects with severe hepatic impairment (Child-Pugh C) compared to subjects with normal hepatic function following a single dose of 960 mg LUMAKRAS.
Clinical Studies
Acid-Reducing Agents: Coadministration of repeat doses of omeprazole (PPI) with a single dose of LUMAKRAS decreased sotorasib Cmax by 65% and AUC by 57% under fed conditions, and decreased sotorasib Cmax by 57% and AUC by 42% under fasted conditions. Coadministration of a single dose of famotidine (H2 receptor antagonist) given 10 hours prior to and 2 hours after a single dose of LUMAKRAS under fed conditions decreased sotorasib Cmax by 35% and AUC by 38%.
Strong CYP3A4 Inducers: Coadministration of repeat doses of rifampin (a strong CYP3A4 inducer) with a single dose of LUMAKRAS decreased sotorasib Cmax by 35% and AUC by 51%.
Other Drugs: No clinically meaningful effect on the exposure of sotorasib was observed following coadministration of LUMAKRAS with itraconazole (a combined strong CYP3A4 and P-gp inhibitor) and a single dose of rifampin (an OATP1B1/1B3 inhibitor), or metformin (a MATE1/MATE2-K substrate).
CYP3A4 substrates: Coadministration of LUMAKRAS with midazolam (a sensitive CYP3A4 substrate) decreased midazolam Cmax by 48% and AUC by 53%.
P-gp substrates: Coadministration of LUMAKRAS with digoxin (a P-gp substrate) increased digoxin Cmax by 91% and AUC by 21%.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with sotorasib.
Sotorasib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay and was not genotoxic in the in vivo rat micronucleus and comet assays.
Fertility/early embryonic development studies were not conducted with sotorasib. There were no adverse effects on female or male reproductive organs in general toxicology studies conducted in dogs and rats.
13.2 Animal Toxicology and/or Pharmacology
In rats, renal toxicity including minimal to marked histologic tubular degeneration/necrosis and increased kidney weight, urea nitrogen, creatinine, and urinary biomarkers of renal tubular injury were present at doses resulting in exposures approximately ≥ 0.5 times the human AUC at the clinical dose of 960 mg. Increases in cysteine S-conjugate β-lyase pathway metabolism in the rat kidney compared to human may make rats more susceptible to renal toxicity due to local formation of a putative sulfur-containing metabolite than humans.
In the 3-month toxicology study in dogs, sotorasib induced findings in the liver (centrilobular hepatocellular hypertrophy), pituitary gland (hypertrophy of basophils), and thyroid gland (marked follicular cell atrophy, moderate to marked colloid depletion, and follicular cell hypertrophy) at exposures approximately 0.4 times the human exposure based on AUC at the clinical dose of 960 mg. These findings may be due to an adaptive response to hepatocellular enzyme induction and subsequent reduced thyroid hormone levels (i.e., secondary hypothyroidism). Although thyroid levels were not measured in dogs, induction of uridine diphosphate glucuronosyltransferase known to be involved in thyroid hormone metabolism was confirmed in the in vitro dog hepatocyte assay.
-
14 CLINICAL STUDIES
14.1 KRAS G12C-mutated Locally Advanced or Metastatic Non-Small Cell Lung Cancer
The efficacy of LUMAKRAS was demonstrated in a subset of patients enrolled in a single-arm, open-label, multicenter trial (CodeBreaK 100 [NCT03600883]). Eligible patients were required to have locally advanced or metastatic KRAS G12C-mutated NSCLC with disease progression after receiving an immune checkpoint inhibitor and/or platinum-based chemotherapy, an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1, and at least one measurable lesion as defined by Response Evaluation Criteria in Solid Tumors (RECIST v1.1).
All patients were required to have prospectively identified KRAS G12C-mutated NSCLC in tumor tissue samples by using the QIAGEN therascreen® KRAS RGQ PCR Kit performed in a central laboratory. Of 126 total enrolled subjects, 2 (2%) were unevaluable for efficacy analysis due to the absence of radiographically measurable lesions at baseline. Of the 124 patients with KRAS G12C mutations confirmed in tumor tissue, plasma samples from 112 patients were tested retrospectively using the Guardant360® CDx. 78/112 patients (70%) had KRAS G12C mutation identified in plasma specimen, 31/112 patients (28%) did not have KRAS G12C mutation identified in plasma specimen and 3/112 (2%) were unevaluable due to Guardant360® CDx test failure.
A total of 124 patients had at least one measurable lesion at baseline assessed by Blinded Independent Central Review (BICR) according to RECIST v1.1 and were treated with LUMAKRAS 960 mg once daily until disease progression or unacceptable toxicity. The major efficacy outcome measures were objective response rate (ORR), and duration of response (DOR) as evaluated by BICR according to RECIST v1.1.
The baseline demographic and disease characteristics of the study population were: median age 64 years (range: 37 to 80) with 48% ≥ 65 years and 8% ≥ 75 years; 50% Female; 82% White, 15% Asian, 2% Black; 70% ECOG PS 1; 96% had stage IV disease; 99% with non-squamous histology; 81% former smokers, 12% current smokers, 5% never smokers. All patients received at least 1 prior line of systemic therapy for metastatic NSCLC; 43% received only 1 prior line of therapy, 35% received 2 prior lines of therapy, 23% received 3 prior lines of therapy; 91% received prior anti-PD-1/PD-L1 immunotherapy, 90% received prior platinum-based chemotherapy, 81% received both platinum-based chemotherapy and anti-PD-1/PD-L1. The sites of known extra-thoracic metastasis included 48% bone, 21% brain, and 21% liver.
Efficacy results are summarized in Table 7.
Table 7. Efficacy Results for Patients with KRAS G12C-mutated NSCLC who Received LUMAKRAS in CodeBreaK 100 Efficacy Parameter LUMAKRAS
N = 124CI = confidence interval - * Assessed by Blinded Independent Central Review (BICR).
- † Estimate using Kaplan-Meier method.
- ‡ Observed proportion of patients with duration of response beyond landmark time.
Objective Response Rate (95% CI)* 36 (28, 45) Complete response rate, % 2 Partial response rate, % 35 Duration of Response* Median†, months (range) 10 (1.3+, 11.1) Patients with duration ≥ 6 months‡, % 58% 14.2 KRAS G12C-mutated Metastatic Colorectal Cancer
The efficacy of LUMAKRAS in combination with panitumumab was evaluated in CodeBreaK 300 [NCT05198934], a multicenter, randomized, open-label, active-controlled study conducted in previously treated patients with KRAS G12C-mutated mCRC. Key eligibility criteria included patients 18 years of age or older, who had received at least one prior line of therapy for mCRC, and who had received fluoropyrimidine, oxaliplatin, and irinotecan for metastatic disease unless there was a medical contraindication.
All patients were also required to have prospectively identified KRAS G12C-mutated mCRC in tumor tissue samples by using the QIAGEN therascreen® KRAS RGQ PCR Kit performed in a central laboratory. Other eligibility criteria included an ECOG PS of ≤ 2 and at least one measurable lesion as defined by RECIST v1.1.
A total of 160 patients with previously treated mCRC with the KRAS G12C mutation were randomized 1:1:1 to receive either LUMAKRAS 960 mg orally once daily and panitumumab 6 mg/kg IV every 2 weeks (N = 53), or LUMAKRAS 240 mg orally once daily and panitumumab 6 mg/kg IV every 2 weeks (N = 53), or investigator's choice of SOC trifluridine/tipiracil or regorafenib (N = 54). Randomization was stratified by prior anti-angiogenic therapy (yes or no), time from initial diagnosis of metastatic disease to randomization (≥ 18 months; < 18 months), and ECOG status (0 or 1 versus 2). Patients received treatment until disease progression, lack of clinical benefit or intolerance to treatment. LUMAKRAS discontinuation required panitumumab discontinuation, however, patients could continue to receive LUMAKRAS if panitumumab was discontinued [see Dosage and Administration (2.3)]. Four patients randomized to LUMAKRAS 960 mg in combination with panitumumab continued LUMAKRAS single agent therapy after discontinuing panitumumab.
The major efficacy outcome measure was progression-free survival (PFS) as evaluated by BICR according to RECIST 1.1. Additional efficacy outcome measures included overall survival (OS), overall response rate (ORR), and duration of response (DOR). Only the results of the approved dosing regimen LUMAKRAS 960 mg in combination with panitumumab are described below.
Of the 107 patients randomized to either LUMAKRAS 960 mg once daily in combination with panitumumab or the control arms, the median age was 64 years (range: 34-81 years); 46% were age 65 years or older; 50% were female; 74% were White; 17% were Asian and 97% of the patients had ECOG PS 0 or 1. The primary site of disease was colon (69%) or rectum (31%). The median number of prior lines of therapy for metastatic disease was 2. Among the 107 patients, 100% received prior fluoropyrimidine, 99% received prior oxaliplatin, 93% received prior irinotecan and 18% of patients had received prior trifluridine and tipiracil, or regorafenib.
The trial demonstrated a statistically significant improvement in PFS for patients randomized to LUMAKRAS 960 mg in combination with panitumumab compared to the investigator's choice SOC. The final analysis of OS was not statistically significant.
The final analysis of PFS for patients randomized to LUMAKRAS 240 mg in combination with panitumumab compared to investigator's choice of SOC was not statistically significant.
The efficacy results from CodeBreaK 300 are summarized in Table 8 and Figure 1.
Table 8. Efficacy Results for Patients with KRAS G12C-mutated mCRC in CodeBreaK 300 Efficacy Parameters Sotorasib 960 mg QD + Panitumumab
(N = 53)SOC (trifluridine/tipiracil or regorafenib)
(N = 54)N = Number of randomized subjects, NR = Not Reached, QD = once daily, SOC = standard of care,
CR = complete response, PR = partial response, BICR = Blinded Independent Central Review Committee,
CI = Confidence Interval- * Hazard ratios and 95% CIs were estimated using a stratified Cox proportional hazards model.
- † p-value was calculated using a stratified log-rank test.
- ‡ OS analysis was based on 6-month additional follow-up data from the time of PFS primary analysis.
- § 95% CIs were estimated using the Clopper-Pearson method.
- ¶ For DOR + indicates censored subjects.
Progression-Free Survival (PFS) per BICR Number of Events (%) 32 (60) 35 (65) Median in months (95% CI) 5.6 (4.2, 6.3) 2 (1.9, 3.9) Hazard ratio (95% CI)* 0.48 (0.3,0.78) p-value (2-sided)† 0.005 Overall Survival (OS)‡ Deaths (%) 24 (45) 30 (56) Median in months (95% CI) NR (8.6, NR) 10.3 (7, NR) Hazard ratio (95% CI)* 0.7 (0.41, 1.18) Overall Response Rate (ORR) per BICR ORR, % (95% CI)§ 26 (15, 40) 0 (0, 7) CR, n (%) 1 (1.9) 0 PR, n (%) 13 (25) 0 Duration of Response (DOR) Median in months, (range)¶ 4.4 (1.9+, 6+) - Figure 1. Kaplan-Meier Curve for Progression-Free Survival in CodeBreaK 300 
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
LUMAKRAS (sotorasib) 320 mg tablets are beige, oval-shaped, film-coated, debossed with "AMG" on one side and "320" on the opposite side, and are supplied as follows:
- Carton containing one bottle of 90 tablets with child-resistant closure, NDC: 55513-504-50
LUMAKRAS (sotorasib) 240 mg tablets are yellow, oval-shaped, film-coated, debossed with "AMG" on one side and "240" on the opposite side, and are supplied as follows:
- Carton containing one bottle of 120 tablets with child-resistant closure, NDC: 55513-512-60
LUMAKRAS (sotorasib) 120 mg tablets are yellow, oblong-shaped, film-coated, debossed with "AMG" on one side and "120" on the opposite side, and are supplied as follows:
- Carton containing two bottles of 120 tablets with child-resistant closure, NDC: 55513-488-02
- Carton containing one bottle of 240 tablets with child-resistant closure, NDC: 55513-488-24
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Hepatotoxicity
Advise patients to immediately contact their healthcare provider for signs and symptoms of liver dysfunction [see Warnings and Precautions (5.1)].
Interstitial Lung Disease (ILD)/Pneumonitis
Advise patients to contact their healthcare provider immediately to report new or worsening respiratory symptoms [see Warnings and Precautions (5.2)].
Lactation
Advise women not to breastfeed during treatment with LUMAKRAS and for 1 week after the last dose [see Use in Specific Populations (8.2)]. Refer to the Full Prescribing Information of panitumumab for lactation information, when LUMAKRAS is used in combination with panitumumab.
Drug Interactions
Advise patients to inform their healthcare provider of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, dietary and herbal products. Inform patients to avoid proton pump inhibitors, and H2 receptor antagonists while taking LUMAKRAS [see Drug Interactions (7.1) and (7.2)].
If coadministration with an acid-reducing agent cannot be avoided, inform patients to take LUMAKRAS 4 hours before or 10 hours after a locally acting antacid [see Dosage and Administration (2.4)].
Missed Dose
If a dose of LUMAKRAS is missed by greater than 6 hours, resume treatment as prescribed the next day [see Dosage and Administration (2.2)].
-
SPL UNCLASSIFIED SECTION
LUMAKRAS in combination with panitumumab
Advise patients taking LUMAKRAS in combination with panitumumab to:
- take the first dose of LUMAKRAS prior to the first panitumumab infusion [see Dosage and Administration (2.2)].
- stop taking panitumumab whenever LUMAKRAS is withheld or discontinued [see Dosage and Administration (2.3)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
LUMAKRAS® (loo-ma-krass)
(sotorasib) tabletsWhat is LUMAKRAS? LUMAKRAS is a prescription medicine used in adults: - alone to treat non-small cell lung cancer (NSCLC):
- that has spread to other parts of the body or cannot be removed by surgery, and
- whose tumor has an abnormal KRAS G12C gene, and
- who have received at least one prior treatment for their cancer.
- in combination with a prescription medicine called panitumumab to treat colon or rectal cancer (CRC):
- that has spread to other parts of the body and
- whose tumor has an abnormal KRAS G12C gene, and
- who have previously received certain chemotherapy medicines.
It is not known if LUMAKRAS is safe and effective in children.
What should I tell my healthcare provider before taking LUMAKRAS?Before taking LUMAKRAS, tell your healthcare provider about all your medical conditions, including if you: - have liver problems.
- have lung or breathing problems other than lung cancer.
- are pregnant or plan to become pregnant. It is not known if LUMAKRAS will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if LUMAKRAS passes into your breast milk. Do not breastfeed during treatment with LUMAKRAS and for 1 week after the last dose.
Especially tell your healthcare provider if you take antacid medicines, including Proton Pump Inhibitor (PPI) medicines or H2 blockers during treatment with LUMAKRAS. Ask your healthcare provider if you are not sure.
How should I take LUMAKRAS?- Take LUMAKRAS exactly as your healthcare provider tells you to take it. Do not change your dose or stop taking LUMAKRAS unless your healthcare provider tells you to.
- Take your prescribed dose of LUMAKRAS 1 time each day, at the same time each day.
- For colon or rectal cancer, you will also receive panitumumab through a vein in your arm (intravenously) given by your healthcare provider. You will first take LUMAKRAS before you receive your first dose of panitumumab. Your healthcare provider will temporarily or permanently stop your treatment with panitumumab if your treatment with LUMAKRAS is temporarily or permanently stopped.
- Take LUMAKRAS with or without food.
- Swallow LUMAKRAS tablets whole. Do not chew, crush, or split tablets.
- If you cannot swallow LUMAKRAS tablets whole:
- Place your prescribed dose of LUMAKRAS in a glass of 4 ounces (120 mL) of non-carbonated, room temperature water without crushing the tablets. Do not use any other liquids.
- Stir or swirl the cup for about 3 minutes until the tablets are in small pieces (the tablets will not completely dissolve). The color of the mixture may be pale yellow to bright yellow.
- Drink the LUMAKRAS and water mixture right away or within 2 hours of preparing. Do not chew pieces of the tablet.
- Rinse the glass with an additional 4 ounces (120 mL) of water and drink to make sure that you have taken the full dose of LUMAKRAS.
- If you do not drink the mixture right away, stir or swirl the mixture again before drinking.
- If you take an antacid medicine, take LUMAKRAS either 4 hours before or 10 hours after the antacid.
- If you miss a dose of LUMAKRAS, take the dose as soon as you remember. If it has been more than 6 hours, do not take the dose. Take your next dose at your regularly scheduled time the next day. Do not take 2 doses at the same time to make up for a missed dose.
- If you vomit after taking a dose of LUMAKRAS, do not take an extra dose. Take your next dose at your regularly scheduled time the next day.
LUMAKRAS may cause serious side effects, including: - Liver problems. Abnormal liver blood tests are common with LUMAKRAS and can sometimes be severe. Your healthcare provider should do blood tests before starting and during treatment with LUMAKRAS to check your liver function. Tell your healthcare provider right away if you develop any signs or symptoms of liver problems, including:
- your skin or the white part of your eyes turns yellow (jaundice)
- dark or "tea-colored" urine
- light-colored stools (bowel movements)
- tiredness or weakness
- nausea or vomiting
- bleeding or bruising
- loss of appetite
- pain, aching, or tenderness on the right side of your stomach-area (abdomen)
- Lung or breathing problems. LUMAKRAS may cause inflammation of the lungs that can lead to death. Tell your healthcare provider or get emergency medical help right away if you have new or worsening shortness of breath, cough, or fever.
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with LUMAKRAS if you develop side effects.
The most common side effects of LUMAKRAS when used alone for NSCLC include:- diarrhea
- muscle or bone pain
- nausea
- tiredness
- cough
- changes in certain blood tests
The most common side effects of LUMAKRAS when used in combination with panitumumab for CRC include: - skin rash
- dry skin
- diarrhea
- mouth sores
- tiredness
- muscle and bone pain
- changes in certain blood tests
These are not all the possible side effects of LUMAKRAS.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Amgen at 1-800-772-6436 (1-800-77-AMGEN).
How should I store LUMAKRAS?- Store LUMAKRAS at room temperature between 68°F to 77°F (20°C to 25°C).
- The bottle has a child-resistant closure.
General information about the safe and effective use of LUMAKRAS.Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use LUMAKRAS for a condition for which it was not prescribed. Do not give LUMAKRAS to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about LUMAKRAS that is written for healthcare professionals.
What are the ingredients in LUMAKRAS?Active Ingredient: sotorasib
Inactive Ingredients: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, and magnesium stearate. Tablet film coating material contains polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, iron oxide yellow and iron oxide red (320 mg tablet only).Manufactured by: Amgen Inc., One Amgen Center Drive, Thousand Oaks, CA 91320-1799 U.S.A.
© 2021, 2023, 2025 Amgen Inc. All rights reserved.
For more information, go to www.LUMAKRAS.com or call 1-800-772-6436 (1-800-77-AMGEN).
This Patient Information has been approved by the U.S. Food and Drug Administration.Revised: 01/2025 [part number] v3 - alone to treat non-small cell lung cancer (NSCLC):
-
PRINCIPAL DISPLAY PANEL - 120 mg Tablet Bottle Carton Label - NDC: 55513-488-02
NDC: 55513-488-02
LUMAKRAS™
(sotorasib) TABLETS120 mg
Each tablet contains 120 mg sotorasib.
Store at 20°C to 25°C (68°F to 77°F). Excursions
permitted from 15°C to 30°C (59°F to 86°F).Recommended Dosage: See Prescribing Information.
AMGEN®

-
PRINCIPAL DISPLAY PANEL - 120 mg Tablet Bottle Carton Label - NDC: 55513-488-24
NDC: 55513-488-24
LUMAKRAS™
(sotorasib) TABLETS120 mg
Each tablet contains 120 mg sotorasib.
Store at 20°C to 25°C (68°F to 77°F).
Excursions permitted from 15°C to 30°C
(59°F to 86°F).Recommended Dosage: See Prescribing
Information.AMGEN®

-
PRINCIPAL DISPLAY PANEL - 320 mg Tablet Bottle Carton Label
NDC: 55513-504-50
LUMAKRAS®
(sotorasib) TABLETS320 mg | New strength
Each tablet contains 320 mg sotorasib.
Store at 20°C to 25°C (68°F to 77°F).
Excursions permitted from 15°C to 30°C
(59°F to 86°F).Recommended Dosage: See Prescribing
Information.AMGEN®
-
PRINCIPAL DISPLAY PANEL - 240 mg Tablet Bottle Carton Label
NDC: 55513-512-60
LUMAKRAS®
(sotorasib) TABLETS240 mg | New strength
Each tablet contains 240 mg sotorasib.
Store at 20°C to 25°C (68°F to 77°F).
Excursions permitted from 15°C to 30°C
(59°F to 86°F).Recommended Dosage: See Prescribing
Information.AMGEN®
-
INGREDIENTS AND APPEARANCE
LUMAKRAS
sotorasib tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55513-488 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOTORASIB (UNII: 2B2VM6UC8G) (SOTORASIB - UNII:2B2VM6UC8G) SOTORASIB 120 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color YELLOW Score no score Shape RECTANGLE Size 16mm Flavor Imprint Code AMG120 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55513-488-02 2 in 1 CARTON 05/28/2021 1 NDC: 55513-488-01 120 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 55513-488-24 1 in 1 CARTON 05/28/2021 2 NDC: 55513-488-40 240 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC: 55513-488-96 1 in 1 CARTON 05/28/2021 3 120 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214665 05/28/2021 LUMAKRAS
sotorasib tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55513-504 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOTORASIB (UNII: 2B2VM6UC8G) (SOTORASIB - UNII:2B2VM6UC8G) SOTORASIB 320 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color BROWN (beige) Score no score Shape OVAL Size 19mm Flavor Imprint Code AMG320 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55513-504-50 1 in 1 CARTON 02/02/2023 1 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214665 02/02/2023 LUMAKRAS
sotorasib tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55513-512 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOTORASIB (UNII: 2B2VM6UC8G) (SOTORASIB - UNII:2B2VM6UC8G) SOTORASIB 240 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color YELLOW Score no score Shape OVAL Size 18mm Flavor Imprint Code AMG240 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55513-512-60 1 in 1 CARTON 06/27/2024 1 120 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214665 06/27/2024 Labeler - Amgen Inc (039976196)
Trademark Results [LUMAKRAS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LUMAKRAS 97248835 not registered Live/Pending |
Amgen Inc. 2022-02-01 |
 LUMAKRAS 90532537 not registered Live/Pending |
Amgen Inc, 2021-02-17 |
 LUMAKRAS 90044720 not registered Live/Pending |
Amgen Inc. 2020-07-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.