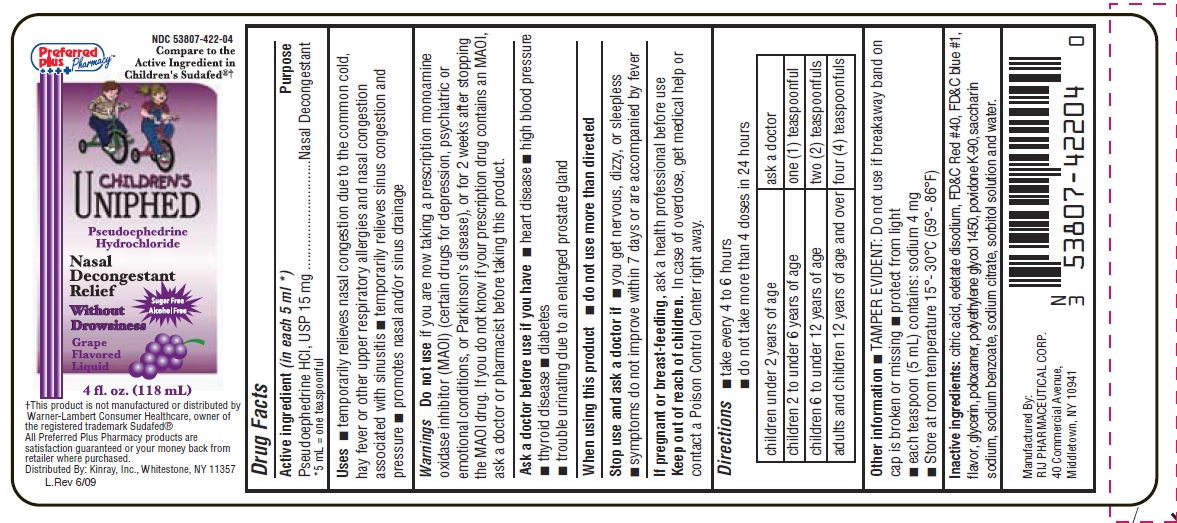
CHILDRENS UNIPHED NASAL DECONGESTANT- pseudoephedrine hydrochloride liquid
Childrens Uniphed by
Drug Labeling and Warnings
Childrens Uniphed by is a Otc medication manufactured, distributed, or labeled by Rij Pharmaceutical Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL*1)
- Purpose
- Uses
-
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHILDRENS UNIPHED NASAL DECONGESTANT
pseudoephedrine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 53807-422 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pseudoephedrine hydrochloride (UNII: 6V9V2RYJ8N) (Pseudoephedrine - UNII:7CUC9DDI9F) Pseudoephedrine hydrochloride 15 mg in 5 mL Inactive Ingredients Ingredient Name Strength anhydrous citric acid (UNII: XF417D3PSL) edetate disodium (UNII: 7FLD91C86K) FD&C blue no. 1 (UNII: H3R47K3TBD) FD&C red no. 40 (UNII: WZB9127XOA) glycerin (UNII: PDC6A3C0OX) poloxamer 407 (UNII: TUF2IVW3M2) polyethylene glycols (UNII: 3WJQ0SDW1A) povidone K90 (UNII: RDH86HJV5Z) water (UNII: 059QF0KO0R) saccharin sodium (UNII: SB8ZUX40TY) sodium benzoate (UNII: OJ245FE5EU) sodium citrate (UNII: 1Q73Q2JULR) sorbitol (UNII: 506T60A25R) Product Characteristics Color PURPLE Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53807-422-04 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/16/1999 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 03/16/1999 Labeler - Rij Pharmaceutical Corporation (144679156) Establishment Name Address ID/FEI Business Operations Rij Pharmaceutical Corporation 144679156 manufacture(53807-422)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.