PlusCBD Sport Recovery Stick 1oz
PlusCBD Sport Recovery Stick 1oz by
Drug Labeling and Warnings
PlusCBD Sport Recovery Stick 1oz by is a Otc medication manufactured, distributed, or labeled by CV Sciences, Inc., Filltech USA, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
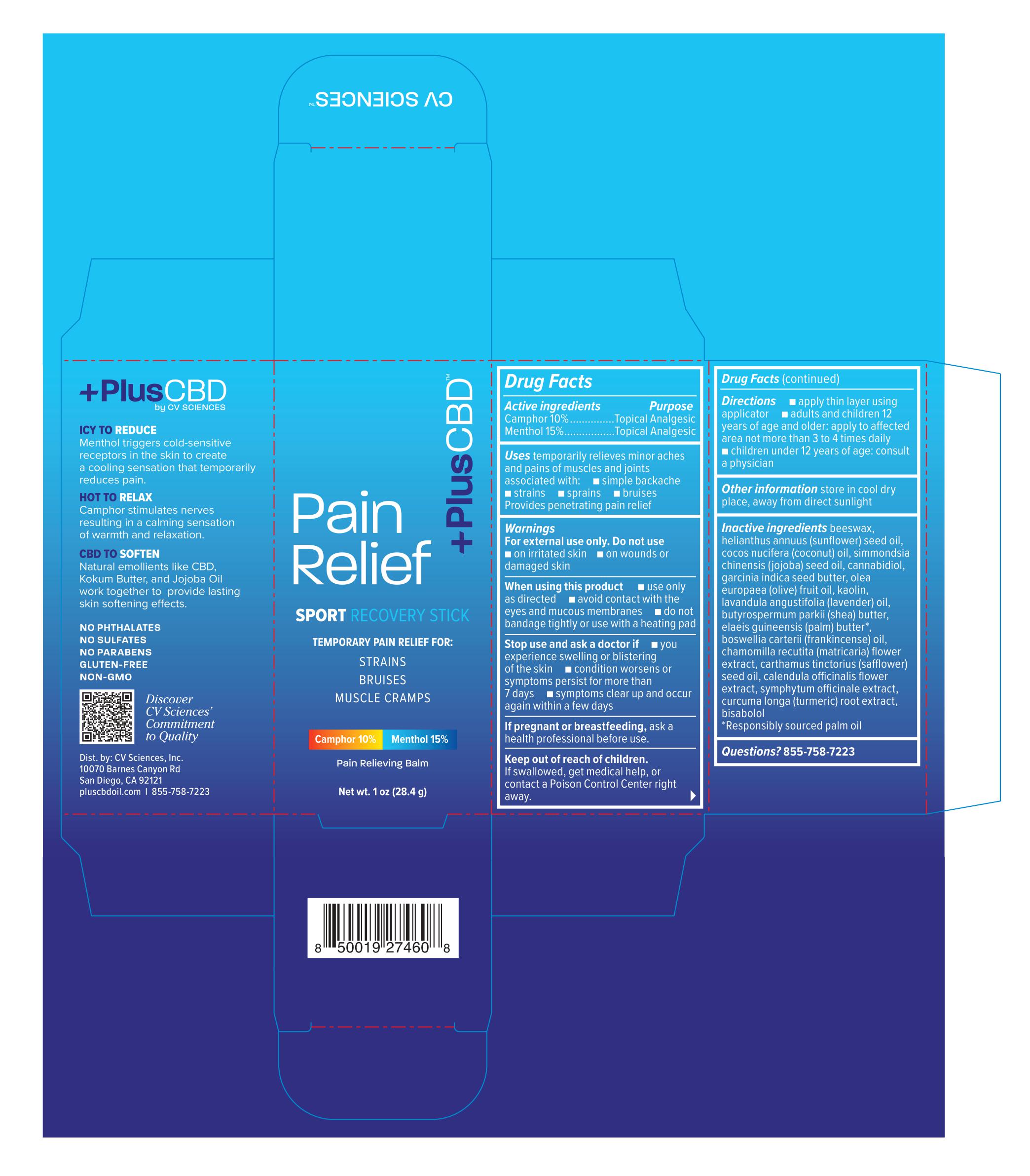
PLUSCBD SPORT RECOVERY STICK 1OZ- camphor, menthol cream
CV Sciences, Inc.
----------
PlusCBD Sport Recovery Stick 1oz
Purpose
Camphor 10%..................Topical Analgesic
Menthol 15%....................Topical Analgesic
Uses
temporarily relieves minor aches and pains of muscles and joints associated with:
- simple backache
- strains
- sprains
- bruises
Provides penetrating pain relief
Warnings
For external use only.
When using this product
- use only as directed
- avoid contact with the eyes and mucous membranes
- do not bandage tightly or use with a heating pad
Directions
- apply thin layer using applicator
- adults and children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: consult a physician
Inactive ingredients
beeswax, helianthus annuus (sunflower) seed oil, cocos nucifera (coconut) oil, simmondsia chinensis (jojoba) seed oil, cannabidiol, garcinia indica seed butter, olea europaea (olive) fruit oil, kaolin, lavandula angustifolia (lavender) oil, butyrospermum parkii (shea) butter, elaeis guineensis (palm) butter*, boswellia carterii (frankincense) oil, chamomilla recutita (matricaria) flower extract, carthamus tinctorius (safflower) seed oil, calendula officinalis flower extract, symphytum officinale extract, curcuma longa (turmeric) root extract, bisbolol
*Responsibly sourced palm oil
| PLUSCBD SPORT RECOVERY STICK 1OZ
camphor, menthol cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - CV Sciences, Inc. (060408113) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Filltech USA, LLC | 926433855 | manufacture(82026-612) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.