CHILDRENS COLD AND ALLERGY- brompheniramine maleate and phenylephrine hcl solution
Childrens Cold and Allergy by
Drug Labeling and Warnings
Childrens Cold and Allergy by is a Otc medication manufactured, distributed, or labeled by Chain Drug Consortium, LLC, Aurohealth LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Purposes
-
Uses
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily relieves these symptoms due to hay fever (allergic rhinitis):
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily restores freer breathing through the nose
-
Warnings
Do not use
- to sedate a child or to make a child sleepy
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- do not take more than 6 doses in any 24-hour period
- measure only with dosage cup provided
- keep dosage cup with product
- do not use any other dosing device
- tsp=teaspoon, mL = milliliter
Age
Dose
adults and children 12 years and over
20 mL (4 tsp) every 4 hours
children 6 to under 12 years
10 mL (2 tsp) every 4 hours
children under 6 years
do not use
- Other information
-
Inactive ingredients
Anhydrous citric acid, FD&C Blue No.1, FD&C Red No. 40, flavor, glycerin, propylene glycol, purified water, sodium benzoate, sodium citrate, sorbitol solution, sucralose
Questions or comments?
1-855-274-4122
*This product is not manufactured or distributed by Pfizer Consumer Healthcare, distributors of children’s Dimetapp® Cold & Allergy
Distributed by:
Chain Drug Consortium, LLC
UPARC, Bldg. A3, Suite 338
1020 William Pitt Way
Pittsburgh, PA 15238
www.chaindrugconsortium.com
MADE IN USA -
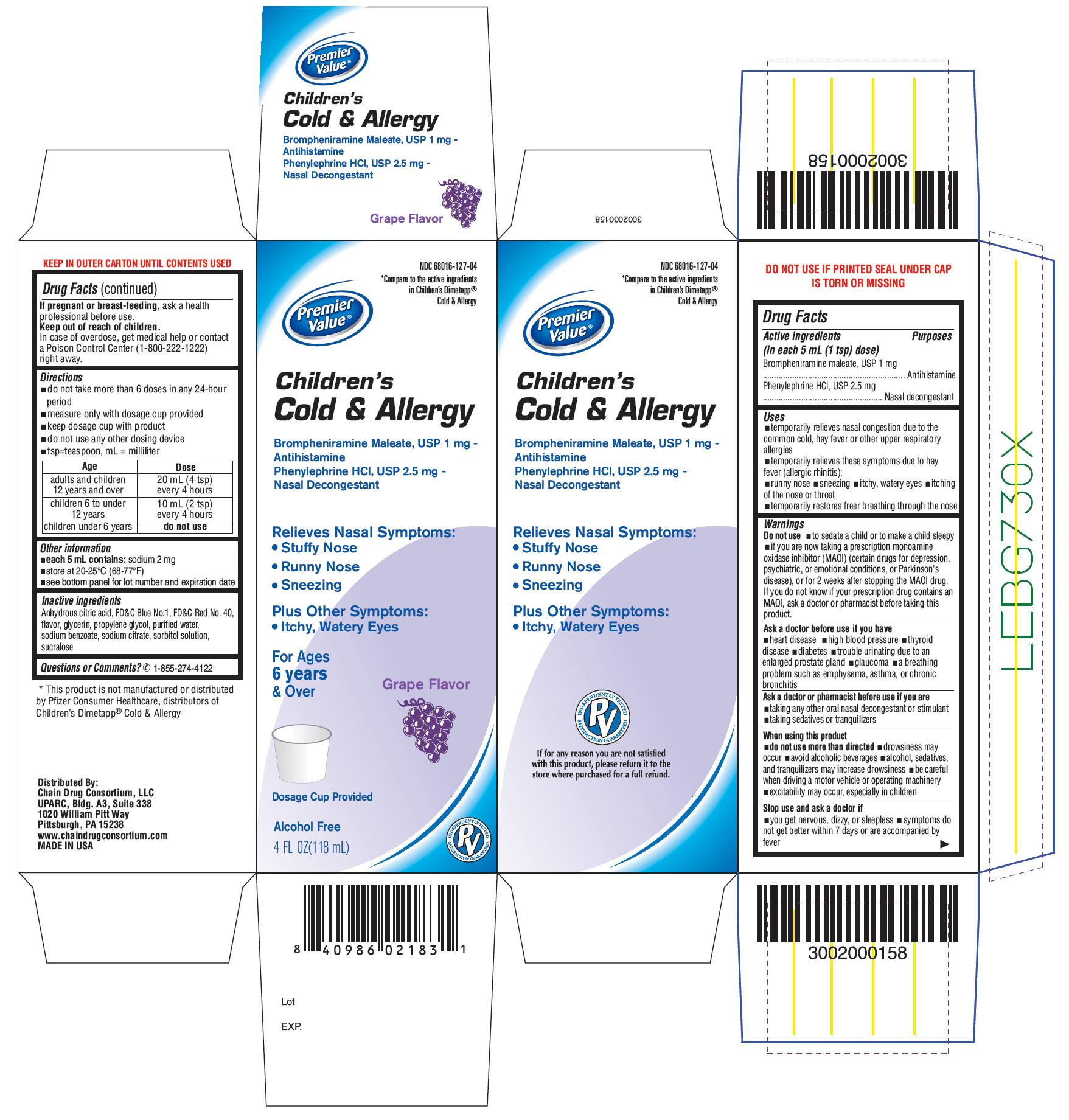
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 4 FL OZ (118 mL Bottle)
NDC: 68016-127-04
*Compare to the active ingredients
in Children's Dimetapp®Cold & Allergy
Premier Value®
Children's
Clod & Allergy
Brompheniramine Maleate, USP 1 mg –
Antihistamine
Phenylephrine HCl, USP 2.5 mg –
Nasal Decongestant
Relieves Nasal Symptoms:- Stuffy Nose
- Runny Nose
- Sneezing
Plus Other Symptoms:
- Itchy, Watery Eyes
For Ages
6 years
& Over
Grape Flavor
Dosage Cup Provided
Alcohol Free
4 FL OZ (118 mL)
-
INGREDIENTS AND APPEARANCE
CHILDRENS COLD AND ALLERGY
brompheniramine maleate and phenylephrine hcl solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68016-127 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BROMPHENIRAMINE MALEATE (UNII: IXA7C9ZN03) (BROMPHENIRAMINE - UNII:H57G17P2FN) BROMPHENIRAMINE MALEATE 1 mg in 5 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GRAPE (UNII: 6X543N684K) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color PURPLE Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68016-127-04 1 in 1 CARTON 06/03/2015 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 06/03/2015 Labeler - Chain Drug Consortium, LLC (101668460) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurohealth LLC 078728447 MANUFACTURE(68016-127)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.