Rose All Day Moisturizer 11041, 71413-210
Rose All Day Moisturizer by
Drug Labeling and Warnings
Rose All Day Moisturizer by is a Otc medication manufactured, distributed, or labeled by Baja Fur, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
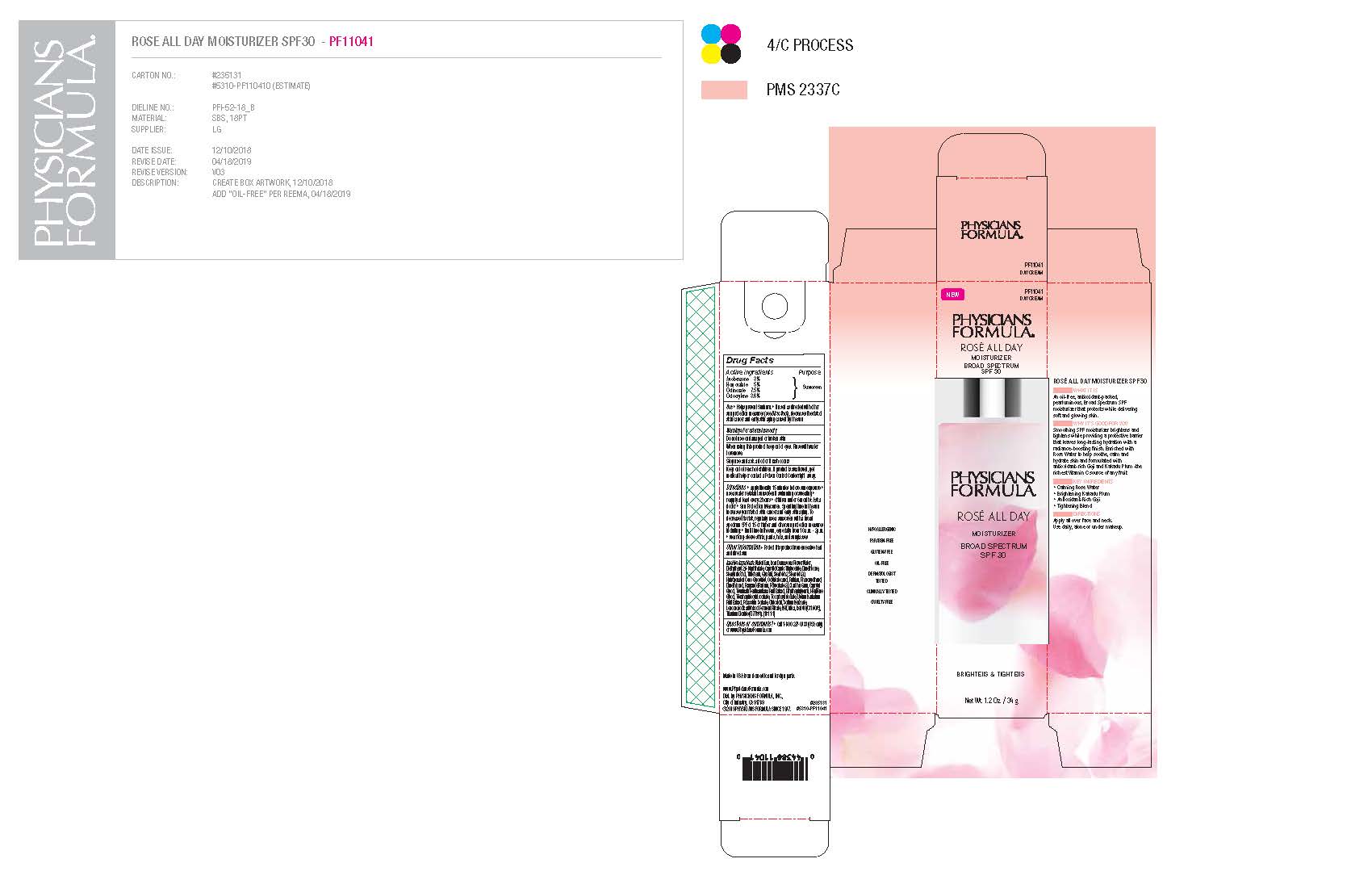
ROSE ALL DAY MOISTURIZER SPF 30- avobenzone, homosalate, octinoxate, octocrylene cream
Baja Fur, S.A. de C.V.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Rose All Day Moisturizer 11041, 71413-210
Uses
-helps prevent sunburn
-if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early dskin aging caused by the sun
Keep out of reach of children
If product is swallowed, get medical help or contact a Poison Control Center right away
Directions
apply liberally 15 minutes before sun exposure use a water resistant sunscreen if swimming or sweating reapply at least every 2 hours children under 6 months: Ask a doctor Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2p.m. wear long-sleeved shirts, pants, hats, and sunglasses
Inactive ingredients
Water/Eau, Rosa Damascena Flower Water, Diethylhexyl 2,6-Naphthalate, Caprylic/Capric Triglyceride, Dimethicone, Stearyl Alcohol, Tribehenin, Glycerin, Steareth-2, Steareth-20, Hydrogenated Coco-Glycerides, Octyldodecanol, Pullulan, Phenoxyethanol, Dimethiconol, Fragrance/Parfum, Polysorbate 20, Xanthan Gum, Caprylyl Glycol, Terminalia Ferdinandiana Fruit Extract, Ethylhexylglycerin, Hexylene Glycol, Tetrahexyldecyl Ascorbate, Tocopheryl Acetate, Lycium Barbarum Fruit Extract, Potassium Sorbate, Citric Acid, Sodium Benzoate, Leuconostoc/Radish Root Ferment Filtrate, BHT, Mica, Red 40 (CI 16035), Titanium Dioxide (CI 77891).
| ROSE ALL DAY MOISTURIZER
SPF 30
avobenzone, homosalate, octinoxate, octocrylene cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Baja Fur, S.A. de C.V. (821533098) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.