ACEBUTOLOL HYDROCHLORIDE CAPSULES
Acebutolol Hydrochloride by
Drug Labeling and Warnings
Acebutolol Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by AvKARE, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ACEBUTOLOL HYDROCHLORIDE- acebutolol hydrochloride capsule
AvKARE, Inc.
----------
ACEBUTOLOL HYDROCHLORIDE CAPSULES
DESCRIPTION
Acebutolol HCl is a selective, hydrophilic beta-adrenoreceptor blocking agent with mild intrinsic sympathomimetic activity for use in treating patients with hypertension and ventricular arrhythmias. It is marketed in capsule form for oral administration. Acebutolol HCl capsules are provided in two dosage strengths which contain 200 or 400 mg of acebutolol as the hydrochloride salt. The inactive ingredients present are D&C Red 28, D&C Yellow 10, FD&C Blue 1, FD&C Red 40, gelatin, maize starch, povidone, titanium dioxide and stearic acid.
Acebutolol HCl has the following structural formula:
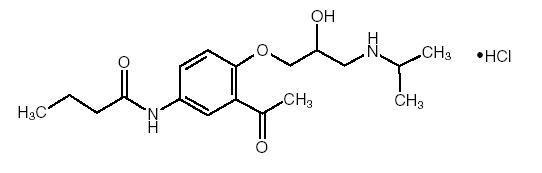
C 18H 28N 2O 4HCl M.W. 372.89
Acebutolol HCl is a white or slightly off-white powder freely soluble in water, and less soluble in alcohol. Chemically it is defined as the hydrochloride salt of (±) N-[3-Acetyl-4-[2-hydroxy-3-[(1-methylethyl) amino]propoxy]phenyl] butanamide.
CLINICAL PHARMACOLOGY
Acebutolol is a cardioselective, β-adrenoreceptor blocking agent, which possesses mild intrinsic sympathomimetic activity (ISA) in its therapeutically effective dose range.
PHARMACODYNAMICS
β 1-cardioselectivity has been demonstrated in experimental animal studies. In anesthetized dogs and cats, acebutolol is more potent in antagonizing isoproterenol-induced tachycardia (β 1) than in antagonizing isoproterenolinduced vasodilatation (β 2). In guinea pigs and cats, it is more potent in antagonizing this tachycardia than in antagonizing isoproterenol-induced bronchodilatation (β 2). ISA of acebutolol has been demonstrated in catecholamine-depleted rats by tachycardia induced by intravenous administration of this agent. A membrane-stabilizing effect has been detected in animals, but only with high concentrations of acebutolol.
Clinical studies have demonstrated β 1-blocking activity at the recommended doses by: a) reduction in the resting heart rate and decrease in exerciseinduced tachycardia; b) reduction in cardiac output at rest and after exercise; c) reduction of systolic and diastolic blood pressures at rest and postexercise; d) inhibition of isoproterenol-induced tachycardia.
The β 1-selectivity of acebutolol has also been demonstrated on the basis of the following vascular and bronchial effects:
Vascular Effects: Acebutolol has less antagonistic effects on peripheral vascular β 2 receptors at rest and after epinephrine stimulation than nonselective β-antagonists.
Bronchial Effects: In single-dose studies in asthmatics examining effects of various beta-blockers on pulmonary function, low doses of acebutolol produce less evidence of bronchoconstriction and less reduction of beta 2 agonist, bronchodilating effects, than nonselective agents like propranolol but more than atenolol.
ISA has been observed with acebutolol in man, as shown by a slightly smaller (about 3 beats per minute) decrease in resting heart rate when compared to equivalent β-blocking doses of propranolol, metoprolol or atenolol. Chronic therapy with acebutolol induced no significant alteration in the blood lipid profile.
Acebutolol has been shown to delay AV conduction time and to increase the refractoriness of the AV node without significantly affecting sinus node recovery time, atrial refractory period, or the HV conduction time. The membrane-stabilizing effect of acebutolol is not manifest at the doses used clinically.
Significant reductions in resting and exercise heart rates and systolic blood pressures have been observed 1.5 hours after acebutolol administration with maximal effects occurring between 3 and 8 hours postdosing in normal volunteers. Acebutolol has demonstrated a significant effect on exercise-induced tachycardia 24 to 30 hours after drug administration.
There are significant correlations between plasma levels of acebutolol and both the reduction in resting heart rate and the percent of β-blockade of exercise-induced tachycardia.
The antihypertensive effect of acebutolol has been shown in double-blind controlled studies to be superior to placebo and similar to propranolol and hydrochlorothiazide. In addition, patients responding to acebutolol administered twice daily had a similar response whether the dosage regimen was changed to once daily administration or continued on a b.i.d. regimen.
Most patients responded to 400 to 800 mg per day in divided doses.
The antiarrhythmic effect of acebutolol was compared with placebo, propranolol, and quinidine. Compared with placebo, acebutolol significantly reduced mean total ventricular ectopic beats (VEB), paired VEB, multiform VEB, R-on-T beats, and ventricular tachycardia (VT). Both acebutolol and propranolol significantly reduced mean total and paired VEB and VT.
Acebutolol and quinidine significantly reduced resting total and complex VEB; the antiarrhythmic efficacy of acebutolol was also observed during exercise.
PHARMACOKINETICS and METABOLISM
Acebutolol is well absorbed from the GI tract. It is subject to extensive first-pass hepatic biotransformation, with an absolute bioavailability of approximately 40% for the parent compound. The major metabolite, an N-acetyl derivative (diacetolol), is pharmacologically active. This metabolite is equipotent to acebutolol and in cats is more cardioselective than acebutolol; therefore, this first-pass phenomenon does not attenuate
the therapeutic effect of acebutolol. Food intake does not have a significant effect on the area under the plasma concentration-time curve (AUC) of acebutolol although the rate of absorption and peak concentration decreased slightly.
The plasma elimination half-life of acebutolol is approximately 3 to 4 hours, while that of its metabolite, diacetolol, is 8 to 13 hours.The time to reach peak concentration for acebutolol is 2.5 hours and for diacetolol, after oral administration of acebutolol, 3.5 hours.
Within the single oral dose range of 200 to 400 mg, the kinetics are dose proportional. However, this linearity is not seen at higher doses, probably due to saturation of hepatic biotransformation sites. In addition, after multiple dosing the lack of linearity is also seen by AUC increases of approximately 100% as compared to single oral dosing. Elimination via renal excretion is approximately 30% to 40% and by nonrenal mechanisms 50% to 60%, which includes excretion into the bile and direct passage through the intestinal wall.
Acebutolol has a low binding affinity for plasma proteins (about 26%). Acebutolol and its metabolite, diacetolol, are relatively hydrophilic and, therefore, only minimal quantities have been detected in the cerebrospinal fluid (CSF).
Drug interaction studies with tolbutamide and warfarin indicated no influence on the therapeutic effects of these compounds. Digoxin and hydrochlorothiazide plasma levels were not affected by concomitant acebutolol administration. The kinetics of acebutolol were not significantly altered by concomitant administration of hydrochlorothiazide, hydralazine, sulfinpyrazone, or oral contraceptives.
In patients with renal impairment, there is no effect on the elimination half-life of acebutolol, but there is decreased elimination of the metabolite, diacetolol, resulting in a two- to three-fold increase in its half-life. For this reason, the drug should be administered with caution in patients with renal insufficiency (see Precautions). Acebutolol and its major metabolite are dialyzable. Acebutolol and its major metabolite are dialyzable. Acebutolol crosses the placental barrier and is secreted in breast milk.
In geriatric patients, the bioavailability of acebutolol and its metabolite is increased, approximately two-fold, probably due to decreases in the first-pass metabolism and renal function in the elderly.
INDICATIONS & USAGE
HYPERTENSION: Acebutolol hydrochloride capsules are indicated for the management of hypertension in adults. It may be used alone or in combination with other antihypertensive agents, especially thiazide-type diuretics.
VENTRICULAR ARRHYTHMIAS: Acebutolol hydrochloride capsules are indicated in the management of ventricular premature beats; it reduces the total number of premature beats, as well as the number of paired and multiform ventricular ectopic beats, and R-on-T beats.
CONTRAINDICATIONS
Acebutolol HCl is contraindicated in: 1) persistently severe bradycardia; 2) second-and third-degree heart block; 3) overt cardiac failure; and 4) cardiogenic shock. (See Warnings.)
WARNINGS
CARDIAC FAILURE: Sympathetic stimulation may be essential for support of the circulation in individuals with diminished myocardial contractility, and its inhibition by β-adrenergic receptor blockade may precipitate more severe failure. Although β-blockers should be avoided in overt cardiac failure, acebutolol can be used with caution in patients with a history of heart failure who are controlled with digitalis and/or diuretics. Both digitalis and acebutolol impair AV conduction. If cardiac failure persists, therapy with acebutolol should be withdrawn.
IN PATIENTS WITHOUT A HISTORY OF CARDIAC FAILURE: In patients with aortic or mitral valve disease or compromised left ventricular function, continued depression of the myocardium with β-blocking agents over a period of time may lead to cardiac failure. At the first signs of failure, patients should be digitalized and/or be given a diuretic and the response observed closely. If cardiac failure continues despite adequate digitalization and/or diuretic, acebutolol therapy should be withdrawn.
EXACERBATION OF ISCHEMIC HEART DISEASE FOLLOWING ABRUPT WITHDRAWAL: Following abrupt cessation of therapy with certain β-blocking agents in patients with coronary artery disease, exacerbation of angina pectoris and, in some cases, myocardial infarction and death have been reported. Therefore, such patients should be cautioned against interruption of therapy without a physician’s advice. Even in the absence of overt ischemic heart disease, when discontinuation of acebutolol is planned, the patient should be carefully observed, and should be advised to limit physical activity to a minimum while acebutolol is gradually withdrawn over a period of about two weeks. (If therapy with an alternative β-blocker is desired, the patient may be transferred directly to comparable doses of another agent without interruption of β-blocking therapy.) If an exacerbation of angina pectoris occurs, antianginal therapy should be restarted immediately in full doses and the patient hospitalized until his condition stabilizes.
PERIPHERAL VASCULAR DISEASE: Treatment with β-antagonists reduces cardiac output and can precipitate or aggravate the symptoms of arterial insufficiency in patients with peripheral or mesenteric vascular disease. Caution should be exercised with such patients, and they should be observed closely for evidence of progression of arterial obstruction.
BRONCHOSPASTIC DISEASES: PATIENTS WITH BRONCHOSPASTIC DISEASE SHOULD, IN GENERAL, NOT RECEIVE A β-BLOCKER. Because of its relative β 1-selectivity, however, low doses of acebutolol may be used with caution in patients with bronchospastic disease who do not respond to, or who cannot tolerate, alternative treatment. Since β 1-selectivity is not absolute and is dose-dependent, the lowest possible dose of acebutolol should be used initially, preferably in divided doses to avoid the higher plasma levels associated with the longer dose-interval. A bronchodilator, such as theophylline or a β 2-stimulant, should be made available in advance with instructions concerning its use.
ANESTHESIA AND MAJOR SURGERY: The necessity, or desirability, of withdrawal of a β-blocking therapy prior to major surgery is controversial. β-adrenergic receptor blockade impairs the ability of the heart to respond to β-adrenergically mediated reflex stimuli. While this might be of benefit in preventing arrhythmic response, the risk of excessive myocardial depression during general anesthesia may be enhanced and difficulty in restarting and maintaining the heart beat has been reported with beta-blockers. If treatment is continued, particular care should be taken when using anesthetic agents which depress the myocardium, such as ether, cyclopropane, and trichlorethylene, and it is prudent to use the lowest possible dose of acebutolol. Acebutolol, like other β-blockers, is a competitive inhibitor of β-receptor agonists, and its effect on the hear t can be reversed by cautious administration of such agents (e.g., dobutamine or isoproterenol – see Overdosage).
Manifestations of excessive vagal tone (e.g., profound bradycardia, hypotension) may be corrected with atropine 1 to 3 mg IV in divided doses.
DIABETES AND HYPOGLYCEMIA: β-blockers may potentiate insulin-induced hypoglycemia and mask some of its manifestations such as tachycardia; however, dizziness and sweating are usually not significantly affected. Diabetic patients should be warned of the possibility of masked hypoglycemia.
THYROTOXICOSIS: β-adrenergic blockade may mask certain clinical signs (tachycardia) of hyperthyroidism. Abrupt withdrawal of β-blockade may precipitate a thyroid storm; therefore, patients suspected of developing thyrotoxicosis from whom acebutolol therapy is to be withdrawn should be monitored closely.
PRECAUTIONS
RISK OF ANAPHYLACTIC REACTION: While taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenged, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
IMPAIRED RENAL OR HEPATIC FUNCTION: Studies on the effect of acebutolol in patients with renal insufficiency have not been performed in the U.S. Foreign published experience shows that acebutolol has been used successfully in chronic renal insufficiency. Acebutolol is excreted through the GI tract, but the active metabolite, diacetolol, is eliminated predominantly by the kidney. There is a linear relationship between renal clearance of diacetolol and creatinine clearance. Therefore, the daily dose of acebutolol should be reduced by 50% when the creatinine clearance is less than 50 mL/min and by 75% when it is less than 25 mL/min. Acebutolol should be used cautiously in patients with impaired hepatic function.
Acebutolol has been used successfully and without problems in elderly patients in the U.S. clinical trials without specific adjustment of dosage. However, elderly patients may require lower maintenance doses because the bioavailability of both acebutolol and its metabolite are approximately doubled in this age group.
INFORMATION FOR PATIENTS: Patients, especially those with evidence of coronary artery disease, should be warned against interruption or discontinuation of acebutolol therapy without a physician’s supervision. Although cardiac failure rarely occurs in properly selected patients, those being treated with β-adrenergic blocking agents should be advised to consult a physician if they develop signs or symptoms suggestive of impending CHF, or unexplained respiratory symptoms.
Patients should also be warned of possible severe hypertensive reactions from concomitant use of α-adrenergic stimulants, such as the nasal decongestants commonly used in OTC cold preparations and nasal drops.
CLINICAL LABORATORY FINDINGS: Acebutolol, like other β-blockers, has been associated with the development of antinuclear antibodies (ANA). In prospective clinical trials, patients receiving acebutolol had a dose-dependent increase in the development of positive ANA titers, and the overall incidence was higher than that observed with propranolol. Symptoms (generally persistent arthralgias and myalgias) related to this laboratory abnormality were infrequent (less than 1% with both drugs). Symptoms and ANA titers were reversible upon discontinuation of treatment.
DRUG INTERACTIONS: Catecholamine-depleting drugs, such as reserpine, may have an additive effect when given with β-blocking agents. Patients treated with acebutolol plus catecholamine depletors should, therefore, be observed closely for evidence of marked bradycardia or hypotension which may present as vertigo, syncope/presyncope, or orthostatic changes in blood pressure without compensatory tachycardia. Exaggerated hypertensive responses have been reported from the combined use of β-adrenergic antagonists and α-adrenergic stimulants, including those contained in proprietary cold remedies and vasoconstrictive nasal drops. Patients receiving β-blockers should be warned of this potential hazard.
Blunting of the antihypertensive effect of beta-adrenoreceptor blocking agents by nonsteroidal anti-inflammatory drugs has been reported.
No significant interactions with digoxin, hydrochlorothiazide, hydralazine, sulfinpyrazone, oral contraceptives, tolbutamide, or warfarin have been observed.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY: Chronic oral toxicity studies in rats and mice, employing dose levels as high as 300 mg/kg/day, which is equivalent to 15 times the maximum recommended (60 kg) human dose, did not indicate a carcinogenic potential for acebutolol. Diacetolol, the major metabolite of acebutolol in man, was without carcinogenic potential in rats when tested at doses as high as 1800 mg/kg/day. Acebutolol and diacetolol were also shown to be devoid of mutagenic potential in the Ames Test. Acebutolol, administered orally to two generations of male and female rats at doses of up to 240 mg/kg/day (equivalent to 12 times the maximum recommended therapeutic dose in a 60-kg human) and diacetolol, administered to two generations of male and female rats at doses of up to 1000 mg/kg/day, had no significant impact on reproductive performance or fertility.
PREGNANCY:
Teratogenic Effects:
Pregnancy Category B: Reproduction studies have been performed with acebutolol in rats (up to 630 mg/kg/day) and rabbits (up to 135 mg/kg/day). These doses are equivalent to approximately 31.5 and 6.8 times the maximum recommended therapeutic dose in a 60-kg human, respectively. The compound was not teratogenic in either species. In the rabbit, however, doses of 135 mg/kg/day caused slight fetal growth retardation; this effect was considered to be a result of maternal toxicity, as evidenced by reduced food intake, a lowered rate of body weight gain, and mortality. Studies have also been performed in these species with diacetolol (at doses of up to 450 mg/kg/day in rabbits and up to 1800 mg/kg/day in rats). Other than a significant elevation in post-implantation loss with 450 mg/kg/day diacetolol, a level at which food consumption and body weight gain were reduced in rabbit dams and a nonstatistically significant increase in incidence of bilateral cataract in rat fetuses from dams treated with 1800 mg/kg/day diacetolol, there was no evidence of harm to the fetus. There are no adequate and well-controlled trials in pregnant women. Because animal teratology studies are not always predictive of the human response, acebutolol should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
Nonteratogenic Effects:
Studies in humans have shown that both acebutolol and diacetolol cross the placenta. Neonates of mothers who have received acebutolol during pregnancy have reduced birth weight, decreased blood pressure, and decreased heart rate. In the newborn the elimination half-life of acebutolol was 6 to 14 hours, while the half-life of diacetolol was 24 to 30 hours for the first 24 hours after birth, followed by a half-life of 12 to 16 hours. Adequate facilities for monitoring these infants at birth should be available.
LABOR AND DELIVERY: The effect of acebutolol on labor and delivery in pregnant women is unknown. Studies in animals have not shown any effect of acebutolol on the usual course of labor and delivery.
NURSING MOTHERS: Acebutolol and diacetolol also appear in breast milk with a milk:plasma ratio of 7.1 and 12.2, respectively. Use in nursing mothers is not recommended.
PEDIATRIC USE: Safety and effectiveness in pediatric patients have not been established.
GERIATRIC USE: Clinical studies of acebutolol and other reported clinical experience is inadequate to determine whether there are differences in safety or effectiveness between patients above or below age 65. Elderly subjects evidence greater bioavailability of acebutolol (see Clinical Pharmacology - PHARMACOKINETICS AND METABOLISM), presumably because of age related reduction in first-pass metabolism and renal function. Therefore, it may be appropriate to start elderly patients at the low end of the dosing range (see Dosage and Administration - USE IN OLDER PATIENTS).
ADVERSE REACTIONS
Acebutolol is well tolerated in properly selected patients. Most adverse reactions have been mild, not required discontinuation of therapy, and tended to decrease as duration of treatment increases.
The following table shows the frequency of treatment-related side effects derived from controlled clinical trials in patients with hypertension, angina pectoris, and arrhythmia. These patients received acebutolol, propranolol, or hydrochlorothiazide as monotherapy, or placebo.
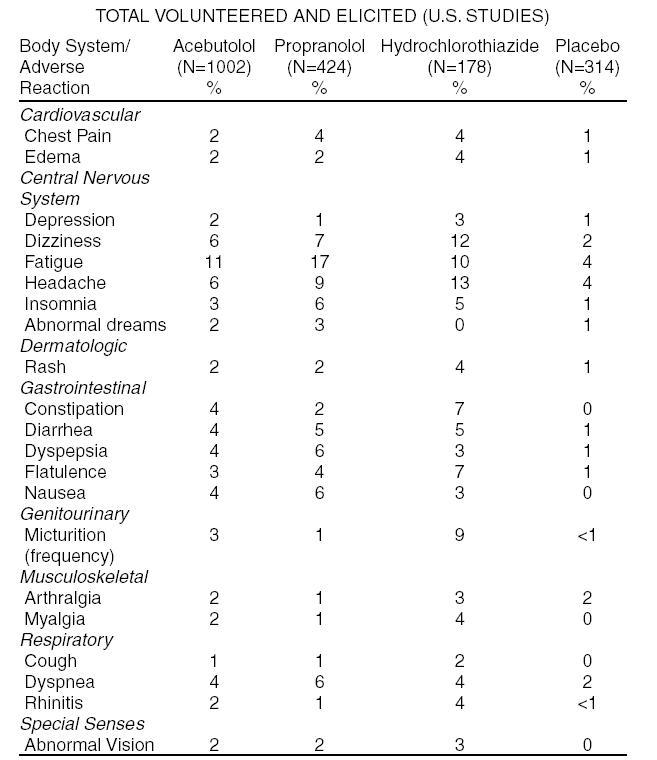
The following selected (potentially important) side effects were seen in up to 2% of acebutolol patients:
Cardiovascular: hypotension, bradycardia, heart failure.
Central Nervous System: anxiety, hyper/hypoesthesia, impotence.
Dermatological: pruritus.
Gastrointestinal: vomiting, abdominal pain.
Genitourinary: dysuria, nocturia.
Liver and Biliary System: A small number of cases of liver abnormalities (increased SGOT, SGPT, LDH) have been reported in association with acebutolol therapy. In some cases increased bilirubin or alkaline phosphatase, fever, malaise, dark urine, anorexia, nausea, headache, and/or other symptoms have been reported. In some of the reported cases, the symptoms and signs were confirmed by rechallenge with acebutolol. The abnormalities were reversible upon cessation of acebutolol therapy.
Musculoskeletal: back pain, joint pain.
Respiratory: pharyngitis, wheezing.
Special Senses: conjunctivitis, dry eye, eye pain.
Autoimmune: In extremely rare instances, systemic lupus erythematosus has been reported.
The incidence of drug-related adverse effects (volunteered and solicited) according to acebutolol dose is shown below. (Data from 266 hypertensive patients treated for 3 months on a constant dose.)
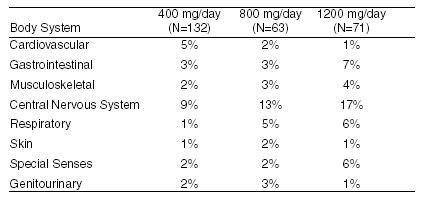
POTENTIAL ADVERSE EFFECTS
In addition, certain adverse effects not listed above have been reported with other β-blocking agents and should also be considered as potential adverse effects of acebutolol.
Central Nervous System: Reversible mental depression progressing to catatonia (an acute syndrome characterized by disorientation for time and place), short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance (neuropsychometrics).
Cardiovascular: Intensification of AV block (see Contraindications).
Allergic: Erythematous rash, fever combined with aching and sore throat, laryngospasm, and respiratory distress.
Hematologic: Agranulocytosis, nonthrombocytopenic, and thrombocytopenic purpura.
Gastrointestinal: Mesenteric arterial thrombosis and ischemic colitis.
Miscellaneous: Reversible alopecia and Peyronie’s disease. The oculomucocutaneous syndrome associated with the β-blocker practolol has not been reported with acebutolol during investigational use and extensive foreign clinical experience.
To report SUSPECTED ADVERSE REACTIONS contact AvKARE, Inc. at 1-855-361-3993; email
drugsafety@avkare.com; or FDA at 1-800-FDA-1088 or
www.fda.gov/medwatch.
OVERDOSAGE
No specific information on emergency treatment of overdosage is available for acebutolol.
However, overdosage with other β-blocking agents has been accompanied by extreme bradycardia, advanced atrioventricular block, intraventricular conduction defects, hypotension, severe congestive heart failure, seizures, and in susceptible patients, bronchospasm and hypoglycemia. Although specific information on the emergency treatment of acebutolol overdose is not available on the basis of the pharmacological actions and the observations in treating overdoses with other β-blockers, the following general measures should be considered:
1. Empty stomach by emesis or lavage.
2. Bradycardia: IV atropine (1 to 3 mg in divided doses). If antivagal response is inadequate, administer isoproterenol cautiously since larger than usual doses of isoproterenol may be required.
3. Persistent hypotension in spite of correction of bradycardia: Administer vasopressor (e.g., epinephrine, norepinephrine, dopamine, or dobutamine) with frequent monitoring of blood pressure and pulse rate.
4. Bronchospasm: A theophylline derivative, such as aminophylline and/or parenteral β 2-stimulant, such as terbutaline.
5. Cardiac failure: Digitalize the patient and/or administer a diuretic. It has been reported that glucagon is useful in this situation.
Acebutolol is dialyzable.
DOSAGE & ADMINISTRATION
HYPERTENSION: The initial dosage of acebutolol in uncomplicated mild-to-moderate hypertension is 400 mg. This can be given as a single daily dose, but in occasional patients twice daily dosing may be required for adequate 24-hour blood-pressure control. An optimal response is usually achieved with dosages of 400 to 800 mg per day, although some patients have been maintained on as little as 200 mg per day. Patients with more severe hypertension or who have demonstrated inadequate control may respond to a total of 1200 mg daily (administered b.i.d.), or to the addition of a second antihypertensive agent. Beta-1 selectivity diminishes as dosage is increased.
VENTRICULAR ARRHYTHMIA: The usual initial dose of acebutolol is 400 mg daily given as 200 mg b.i.d. Dosage should be increased gradually until an optimal clinical response is obtained, generally at 600 to 1200 mg per day. If treatment is to be discontinued, the dosage should be reduced gradually over a period of about two weeks.
USE IN OLDER PATIENTS: Older patients have an approximately 2-fold increase in bioavailability and may require lower maintenance doses. Doses above 800 mg/day should be avoided in the elderly.
HOW SUPPLIED
Acebutolol hydrochloride capsules are available as follows:
200 mg: Size "2" hard gelatin capsules with bright orange opaque body printed radially "669" with black ink and lavender opaque cap printed radially "Amneal" with black ink.
NDC: 42291-101-90 Bottles of 90
400 mg: Size "2" hard gelatin capsules with bright orange opaque body printed radially "670" with black ink and lavender opaque cap printed radially "Amneal" with black ink.
NDC: 42291-102-90 Bottles of 90
Store at 20° to 25°C (68° to 77°F) (See USP Controlled Room Temperature).
Keep tightly closed. Dispense in a light resistant, tight container.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
AvKARE
NDC: 42291-101-90
Acebutolol Hydrochloride Capsules
200 mg
90 Capsules Rx Only
Each capsule contains: acebutolol hydrochloride equivalent to 200 mg of acebutolol.
USUAL ADULT DOSAGE: See package insert for full prescribing information.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN
Dispense in a tight, light-resistant container. Keep tightly closed.
Store at 20° to 25°C (68°–77°F) (See USP Controlled Room Temperature). Protect from light.
Manufactured for:
AvKARE Inc.
Pulaski, TN 38478
Mfg. Rev. 07/10 AV Rev. 10/13 (P)
N3 42291-10190
6

AvKARE
NDC: 42291-102-90
Acebutolol Hydrochloride Capsules
400mg
90 Capsules Rx Only
Each capsule contains: acebutolol hydrochloride equivalent to 400 mg of acebutolol.
USUAL ADULT DOSAGE: See package insert for full prescribing information.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN
Dispense in a tight, light-resistant container. Keep tightly closed.
Store at 20° to 25°C (68°–77°F) (See USP Controlled Room Temperature). Protect from light.
Manufactured for:
AvKARE Inc.
Pulaski, TN 38478
Mfg. Rev. 09/10 AV Rev. 02/15 (P)
N3 42291 10290
3

| ACEBUTOLOL HYDROCHLORIDE
acebutolol hydrochloride capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| ACEBUTOLOL HYDROCHLORIDE
acebutolol hydrochloride capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - AvKARE, Inc. (796560394) |