PRENATE ENHANCE- ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, folic acid, pyridoxine hydrochloride, cyanocobalamin, biotin, calcium carbonate, ferrous fumarate, magnesium oxide, lactic acid and doconexent capsule, gelatin coated
PRENATE Enhance by
Drug Labeling and Warnings
PRENATE Enhance by is a Prescription medication manufactured, distributed, or labeled by Avion Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- INACTIVE INGREDIENT
-
INDICATIONS & USAGE
INDICATIONS: PRENATE ® Enhance is a multivitamin/multimineral fatty acid dietary supplement indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers. PRENATE ® Enhance can also be beneficial in improving the nutritional status of women prior to conception.
- CONTRAINDICATIONS
-
BOXED WARNING
(What is this?)
WARNING: Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
-
PRECAUTIONS
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
- BOXED WARNING (What is this?)
- ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
-
HOW SUPPLIED
HOW SUPPLIED: Bottles of 30 softgels (75854-309-30). The listed product number is not a National Drug Code. Instead, Avion Pharmaceuticals has assigned a product code formatted according to standard industry practice to meet the formatting requirements of pharmacy and healthcare insurance computer systems.
- STORAGE AND HANDLING
- WARNINGS
-
SPL UNCLASSIFIED SECTION
MANUFACTURED FOR:
Avion Pharmaceuticals, LLC
Atlanta, GA 30005
1-888-61-AVIONRev. 0319-01
Formical® is a registered trademark of Nephro-Tech 1, LLC, covered by one or more claims of U.S. Patent No. 6,528,542.
Sumalate® is a registered trademark of Albion Laboratories, Inc., covered by one or more claims of U.S. Patent Nos. 5,516,925, 6,716,814, 8,007,846 and 8,425,956.
THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE
- PRINCIPAL DISPLAY PANEL - 30 Softgel label
-
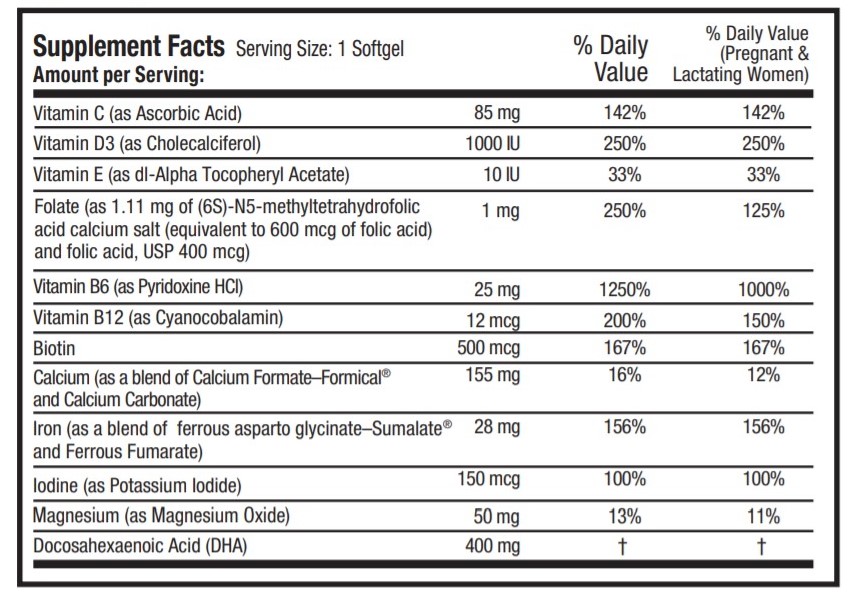
INGREDIENTS AND APPEARANCE
PRENATE ENHANCE
ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, folic acid, pyridoxine hydrochloride, cyanocobalamin, biotin, calcium carbonate, ferrous fumarate, magnesium oxide, lactic acid and doconexent capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 75854-309 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 85 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 1000 [iU] .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 10 [iU] FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 25 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 500 ug CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 155 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 28 mg POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION 150 mg MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 50 mg DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 400 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) WHITE WAX (UNII: 7G1J5DA97F) CORN OIL (UNII: 8470G57WFM) WATER (UNII: 059QF0KO0R) FD&C RED NO. 40 (UNII: WZB9127XOA) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color purple (dark purple) Score no score Shape OVAL Size 22mm Flavor Imprint Code N Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75854-309-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/16/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/17/2013 Labeler - Avion Pharmaceuticals, LLC (040348516) Establishment Name Address ID/FEI Business Operations Avion Pharmaceuticals, LLC 040348516 manufacture(75854-309)
Trademark Results [PRENATE Enhance]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PRENATE ENHANCE 86818419 5089391 Live/Registered |
AVION PHARMACEUTICALS, LLC 2015-11-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.