ACETAMINOPHEN suspension
ACETAMINOPHEN by
Drug Labeling and Warnings
ACETAMINOPHEN by is a Otc medication manufactured, distributed, or labeled by CHAIN DRUG MARKETING ASSOCIATION INC, Seaway Pharma Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
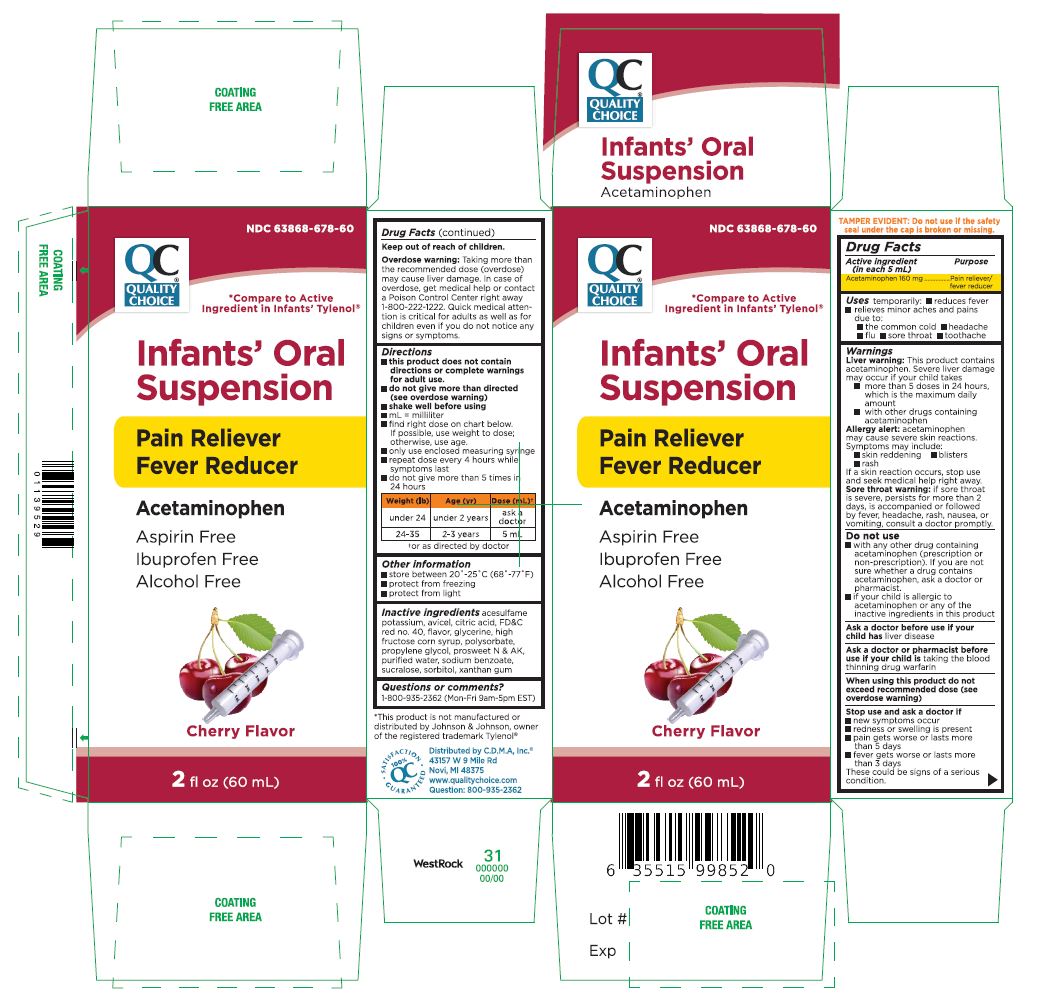
- Active Ingredient Purpose(in each 5 mL)
- PURPOSE
- Uses temporarily:
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or
vomiting, consult a doctor promptly.When using this product do not exceed recommended dose (see overdose warning)
- Do not use
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- Stop use and ask a doctor if
- KEEP OUT OF REACH OF CHILDREN
-
OVERDOSAGE
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away 1-800-222-1222. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
-
Directions
- this product does not contain directions or complete warnings for adult use.
- do not give more than directed (see overdose warning)
- shake well before using
- ml= milliliter
- find right dose on chart below.
If possible, use weight to dose; otherwise, use age.
- only use enclosed measuring syringe
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63868-678 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 5 mL Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) SORBITOL (UNII: 506T60A25R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63868-678-60 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 02/01/2021 Labeler - CHAIN DRUG MARKETING ASSOCIATION INC (011920774) Registrant - Seaway Pharma Inc. (117218785) Establishment Name Address ID/FEI Business Operations Seaway Pharma Inc. 117218785 manufacture(63868-678)
Trademark Results [ACETAMINOPHEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACETAMINOPHEN 85615223 not registered Dead/Abandoned |
General Merchandise importers and Expoters 2012-05-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.