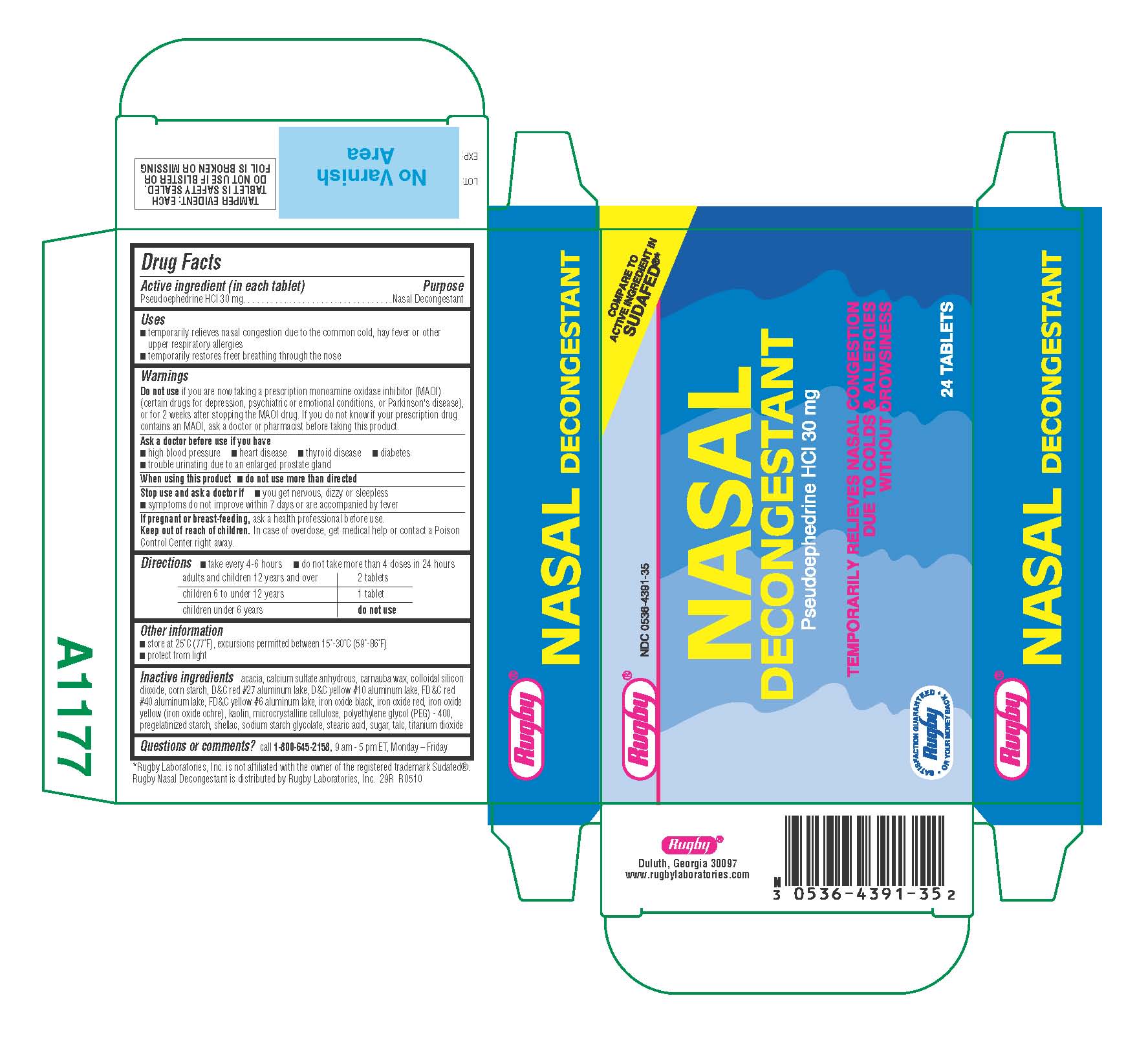
Nasal Decongestant by Rugby Laboratories, Inc / Time Cap Labs, Inc RUGBY 29R
Nasal Decongestant by
Drug Labeling and Warnings
Nasal Decongestant by is a Otc medication manufactured, distributed, or labeled by Rugby Laboratories, Inc, Time Cap Labs, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NASAL DECONGESTANT- pseudoephedrine hcl tablet, sugar coated
Rugby Laboratories, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
RUGBY 29R
|
Uses
|
|
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. |
|
Directions
children 6 to under 12 years: 1 tablet children under 6 years: do not use |
|
Inactive ingredients acacia, calcium sulfate anhydrous, carnauba wax, colloidal silicon dioxide, corn starch, D-C red #27 aluminum lake, D-C yellow #10 aluminum lake, FD-C red #40 aluminum lake, FD-C yellow #6 aluminum lake, iron oxide black, iron oxide red, iron oxide yellow (iron oxide ochre), kaolin, microcrystalline cellulose, polyethylene glycol (PEG) - 400, pregelatinized starch, shellac, sodium starch glycolate, stearic acid, sugar, talc, titanium dioxide |
| NASAL DECONGESTANT
pseudoephedrine hcl tablet, sugar coated |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Rugby Laboratories, Inc (079246066) |
| Registrant - Time Cap Labs, Inc (037052099) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Time Cap Labs, Inc | 037052099 | manufacture(0536-4391) | |