CHILDRENS COLD, COUGH AND SORE THROAT- acetaminophen, dextromethorphan hydrobromide, guaifenesin and phenylephrine hydrochloride solution
Childrens Cold, Cough and Sore Throat by
Drug Labeling and Warnings
Childrens Cold, Cough and Sore Throat by is a Otc medication manufactured, distributed, or labeled by Aurohealth LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Purposes
-
Uses
- temporarily relieves these common cold and flu symptoms:
- nasal congestion
- stuffy nose
- cough due to minor throat and bronchial irritation
- the intensity of coughing
- the impulse to cough to help your child get to sleep
- minor aches and pains
- sore throat
- headache
- temporarily reduces fever
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves these common cold and flu symptoms:
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting, consult a doctor promptly.
-
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
- Ask a doctor before use if your child has
- Ask a doctor or pharmacist before use if
- When using this product
-
Stop use and ask a doctor if
- nervousness, dizziness or sleeplessness occur
- pain, nasal congestion or cough gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back, or occurs with rash or headache that lasts. These could be signs of a serious condition.
- Keep out of reach of children.
-
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed (see Overdose warning)
- do not give more than 5 doses in any 24-hour period
- if needed, repeat dose every 4 hours while symptoms lasts
- do not give more than 5 days unless directed by a doctor
- shake well before using
- measure only with dosing cup provided
- do not use dosing cup with other products
- dose as follows or as directed by a doctor
- mL = milliliter; tsp = teaspoon
- children 6 to under 12 years of age: 10 mL in dosing cup provided
- children under 6 years of age: do not use
- Other information
- Inactive ingredients
- Questions or Comments?
-
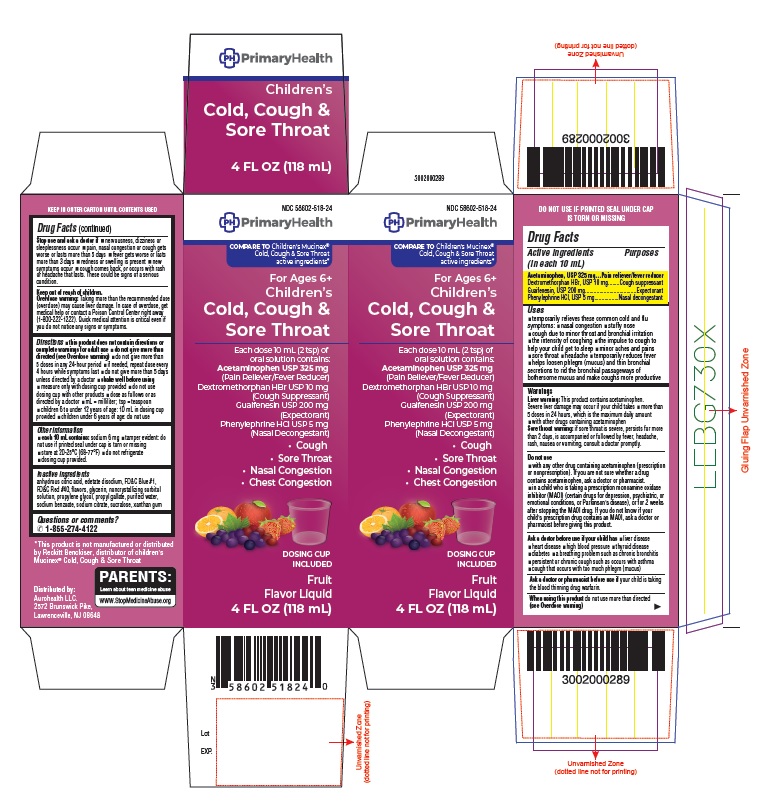
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL -4 FL OZ (118 mL Container Label)
NDC: 58602-518-24
PrimaryHealth
For Ages 6+
Children's
Cold, Cough, &
Sore Throat
Each dose 10 mL (2 tsp) of
oral solution contains:
Acetaminophen USP 325 mg
(Pain Reliever/Fever Reducer)
Dextromethorphan HBr USP 10 mg
(Cough Suppressant)
Guaifenesin USP 200 mg
(Expectorant)
Phenylephrine HCl USP 5 mg
(Nasal Decongestant)
- Cough
- Sore Throat
- Nasal Congestion
- Chest Congestion
Fruit
Flavor Liquid
4 FL OZ (118 mL)

-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL -4 FL OZ (118 mL Container Carton Label)
NDC: 58602-518-24
PrimaryHealth
COMPARE TO Children's Mucinex®
Cold, Cough & Sore Throat
active ingredients*
For Ages 6+
Children's
Cold, Cough, &
Sore Throat
Each dose 10 mL (2 tsp) of
oral solution contains:
Acetaminophen USP 325 mg
(Pain Reliever/Fever Reducer)
Dextromethorphan HBr USP 10 mg
(Cough Suppressant)
Guaifenesin USP 200 mg
(Expectorant)
Phenylephrine HCl USP 5 mg
(Nasal Decongestant)-
Cough
-
Sore Throat
-
Nasal Congestion
-
Chest Congestion
DOSING CUP
INCLUDED
Fruit
Flavor Liquid
4 FL OZ (118 mL)
-
-
INGREDIENTS AND APPEARANCE
CHILDRENS COLD, COUGH AND SORE THROAT
acetaminophen, dextromethorphan hydrobromide, guaifenesin and phenylephrine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58602-518 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg in 10 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 10 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 10 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg in 10 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) PINEAPPLE (UNII: 2A88ZO081O) MANGO (UNII: I629I3NR86) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL (UNII: 506T60A25R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYL GALLATE (UNII: 8D4SNN7V92) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color BLUE (Blue to Bluish Green) Score Shape Size Flavor BERRY, MANGO, PINEAPPLE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58602-518-24 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/03/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 05/03/2016 Labeler - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurohealth LLC 078728447 MANUFACTURE(58602-518)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.