VITAFOL CAPLET- vitamin a, ascorbic acid, vitamin d, .alpha.-tocopherol, thiamine mononitrate, riboflavin, niacin, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium, and iron capsule
Vitafol by
Drug Labeling and Warnings
Vitafol by is a Prescription medication manufactured, distributed, or labeled by Exeltis USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
COMPOSITION
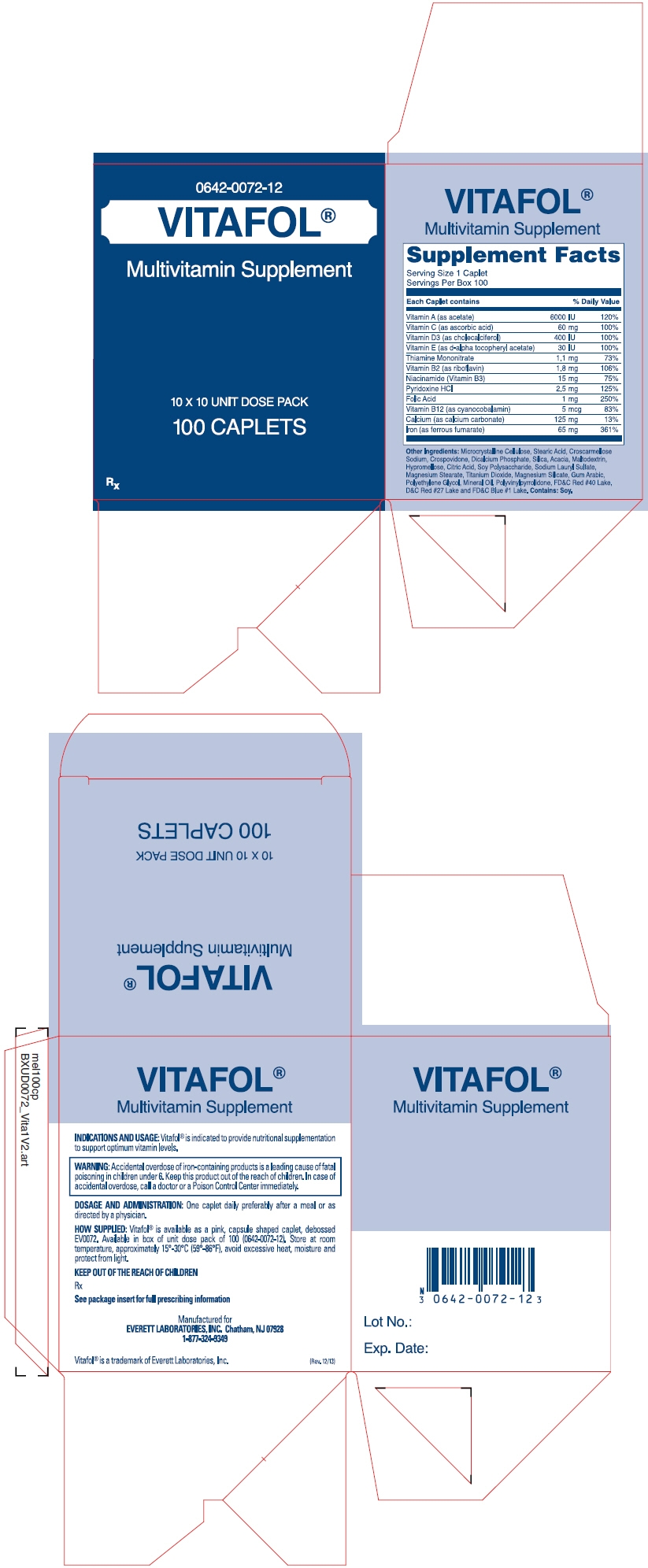
Each caplet contains: Vitamin A (as acetate) 6000 IU Vitamin C (as ascorbic acid) 60 mg Vitamin D-3 (as cholecalciferol) 400 IU Vitamin E (as d-alpha tocopheryl acetate) 30 IU Thiamine Mononitrate 1.1 mg Vitamin B-2 (as riboflavin) 1.8 mg Niacinamide (Vitamin B-3) 15 mg Pyridoxine HCl 2.5 mg Folic Acid 1 .0 mg Vitamin B-12 (as cyanocobalamin) 5 mcg Calcium (as calcium carbonate) 125 mg Iron (as ferrous fumarate) 65 mg Other Ingredients: Microcrystalline Cellulose, Stearic Acid, Croscarmellose Sodium, Crospovidone, Dicalcium Phosphate, Silica, Acacia, Maltodextrin, Hypromellose, Citric Acid, Soy Polysaccharide, Sodium Lauryl Sulfate, Magnesium Stearate, Titanium Dioxide, Magnesium Silicate, Gum Arabic, Polyethylene Glycol, Mineral Oil, Polyvinylpyrrolidone, FD&C Red #40 Lake, D&C Red #27 Lake and FD&C Blue #1 Lake. Contains Soy.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Vitafol® is contraindicated in patients with hypersensitivity to any of its components or color additives. Folic acid is contraindicated in patients with untreated and uncomplicated pernicious anemia, and in those with anaphylactic sensitivity to folic acid. Iron therapy is contraindicated in patients with hemochromatosis and patients with iron storage disease or the potential for iron storage disease due to chronic hemolytic anemia (e.g., inherited anomalies of hemoglobin structure or synthesis and/or red cell enzyme deficiencies, etc.); pyridoxine responsive anemia, or cirrhosis of the liver. Cyanocobalamin is contraindicated in patients with sensitivity to cobalt or to cyanocobalamin (Vitamin B-12).
-
WARNINGS/PRECAUTIONS
Vitamin D supplementation should be used with caution in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. High doses of vitamin D can lead to elevated levels of calcium that reside in the blood and soft tissues. Bone pain, high blood pressure, formation of kidney stones, renal failure, and increased risk of heart disease can occur. Prolonged therapeutic use of iron salts may produce iron storage disease.
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or a Poison Control Center immediately.
Folic acid, especially in doses above 0.1 mg daily, may obscure pernicious anemia, in that hematologic remission may occur while neurological manifestations remain progressive. The use of folic acid doses above 1 mg daily may precipitate or exacerbate the neurological damage of vitamin B12 deficiency.
Avoid overdosage. Keep out of the reach of children.
Drug Interactions
High doses of folic acid may result in decreased serum levels of the anticonvulsant drugs.
Vitamin D supplementation should not be given with large amounts of calcium in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones.
Consult appropriate references for additional specific vitamin-drug interactions.
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Vitafol® is available as a pink, capsule shaped caplet, debossed EV0072. Available in box of unit dose pack of 100 (0642-0072-12).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 100 Caplet Carton
-
INGREDIENTS AND APPEARANCE
VITAFOL CAPLET
vitamin a, ascorbic acid, vitamin d, .alpha.-tocopherol, thiamine mononitrate, riboflavin, niacin, pyridoxine hydrochloride, folic acid, cyanocobalamin, calcium, and iron capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0642-0072 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Vitamin A (UNII: 81G40H8B0T) (Vitamin A - UNII:81G40H8B0T) Vitamin A 6000 [iU] Ascorbic Acid (UNII: PQ6CK8PD0R) (Ascorbic Acid - UNII:PQ6CK8PD0R) Ascorbic Acid 60 mg Vitamin D (UNII: 9VU1KI44GP) (Cholecalciferol - UNII:1C6V77QF41) Vitamin D 400 [iU] .Alpha.-Tocopherol (UNII: H4N855PNZ1) (.Alpha.-Tocopherol - UNII:H4N855PNZ1) .Alpha.-Tocopherol 10 [iU] Thiamine Mononitrate (UNII: 8K0I04919X) (Thiamine Ion - UNII:4ABT0J945J) Thiamine 1.1 mg Riboflavin (UNII: TLM2976OFR) (Riboflavin - UNII:TLM2976OFR) Riboflavin 1.8 mg Niacin (UNII: 2679MF687A) (Niacin - UNII:2679MF687A) Niacin 15 mg Pyridoxine Hydrochloride (UNII: 68Y4CF58BV) (Pyridoxine - UNII:KV2JZ1BI6Z) Pyridoxine Hydrochloride 15 mg Folic Acid (UNII: 935E97BOY8) (Folic Acid - UNII:935E97BOY8) Folic Acid 1 mg Cyanocobalamin (UNII: P6YC3EG204) (Cyanocobalamin - UNII:P6YC3EG204) Cyanocobalamin 5 ug Calcium (UNII: SY7Q814VUP) (Calcium - UNII:SY7Q814VUP) Calcium 125 mg Iron (UNII: E1UOL152H7) (Iron - UNII:E1UOL152H7) Iron 27 mg Inactive Ingredients Ingredient Name Strength Cellulose, Microcrystalline (UNII: OP1R32D61U) Stearic Acid (UNII: 4ELV7Z65AP) Croscarmellose Sodium (UNII: M28OL1HH48) Crospovidone (UNII: 68401960MK) Calcium Phosphate, Dibasic, Anhydrous (UNII: L11K75P92J) Silicon Dioxide (UNII: ETJ7Z6XBU4) Acacia (UNII: 5C5403N26O) Maltodextrin (UNII: 7CVR7L4A2D) Hypromelloses (UNII: 3NXW29V3WO) Citric Acid Monohydrate (UNII: 2968PHW8QP) Lecithin, Soybean (UNII: 1DI56QDM62) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycols (UNII: 3WJQ0SDW1A) Mineral Oil (UNII: T5L8T28FGP) Povidone K30 (UNII: U725QWY32X) FD&C Red No. 40 (UNII: WZB9127XOA) D&C Red No. 27 (UNII: 2LRS185U6K) FD&C Blue No. 1 (UNII: H3R47K3TBD) Product Characteristics Color PINK Score no score Shape CAPSULE Size 19mm Flavor Imprint Code EV0072 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0642-0072-12 100 in 1 BOX; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 05/01/1985 Labeler - Everett Laboratories, Inc. (071170534)
Trademark Results [Vitafol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VITAFOL 88294273 not registered Live/Pending |
EXELTIS USA INC. 2019-02-08 |
 VITAFOL 86653118 4859841 Live/Registered |
Exeltis USA, Inc. 2015-06-05 |
 VITAFOL 73611865 1452861 Live/Registered |
EVERETT LABORATORIES, INC. 1986-07-28 |
 VITAFOL 72084824 0728602 Live/Registered |
VITA ZAHNFABRIK H. RAUTER, KG 1959-11-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.