QUALITY CHOICE NAUSEA RELIEF- sodium citrate dihydrate tablet, chewable
QUALITY CHOICE by
Drug Labeling and Warnings
QUALITY CHOICE by is a Otc medication manufactured, distributed, or labeled by QUALITY CHOICE (CHAIN DRUG MARKETING ASSOCIATION). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
- Warnings
- DO NOT USE
- Ask a doctor before use if you
- ASK DOCTOR/PHARMACIST
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Adults: 2-4 tablets.
- Children: Consult a doctor for appropriate dosage.
- Chew tablets completely. Do not swallow tablet whole.
- Dosage may be repeated after 15 minutes, not to exceed 24 tablets in a 24-hour period unless advised by a doctor.
- Read all package directions and warnings before use and use only as directed.
- These tablets are intended for use by normally healthy persons only.
- Persons under 18 years of age should use only as directed by a doctor.
-
Other information
- Each tablet contains:sodium 60 mg
- Store at room temperature.
- Protect product from excessive heat, cold and moisture.
- Note: This product is not intended as a substitute for a balanced nutritional diet or as an electrolyte replenishment.
- You may report serious side effects to the phone number provided under Questions?
- Inactive ingredients
- Questions
-
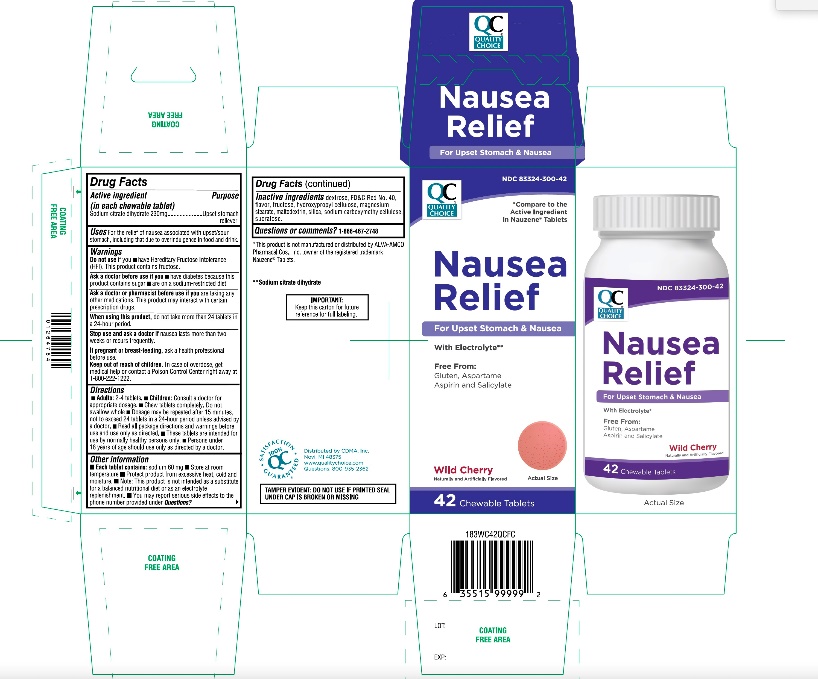
Principal Display Panel
Quality Choice®
*Compare to the Active Ingredient in Nauzene® Tablets
NDC# 83324-300-42
Nausea Relief
For upset stomach & nausea
With electrolyte**
Free From:
Gluten, Aspartame
Aspirin and Salicylate
Wild Cherry Flavor
Naturally & Artificially Flavored
42 CHEWABLE TABLETS
TAMPER EVIDENT: DO NOT USE IF PRINTED SEAL UNDER CAP IS BROKEN OR MISSING
IMPORTANT: Keep this carton for future reference on full labeling
Distributed by: CDMA, Inc.
Novi, MI 48375
Questions: 800-935-2362
100% SATISFACTION GUARANTEED
*This product is not manufactured of distributed by ALVA-AMCO Pharmacal Cos., Inc., the distributor of Nauzene® Tablets.
**Sodium citrate dihydrate
-
INGREDIENTS AND APPEARANCE
QUALITY CHOICE NAUSEA RELIEF
sodium citrate dihydrate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 83324-300 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 230 mg Inactive Ingredients Ingredient Name Strength DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) CHERRY (UNII: BUC5I9595W) FRUCTOSE (UNII: 6YSS42VSEV) HYDROXYPROPYL CELLULOSE (110000 WAMW) (UNII: 5Y0974F5PW) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color pink Score no score Shape ROUND Size 16mm Flavor CHERRY (Wild Cherry Flavor) Imprint Code RP183 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83324-300-42 3 in 1 CARTON 09/15/2025 1 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/15/2025 Labeler - QUALITY CHOICE (CHAIN DRUG MARKETING ASSOCIATION) (011920774)
Trademark Results [QUALITY CHOICE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 QUALITY CHOICE 77326160 3440520 Live/Registered |
CHAIN DRUG MARKETING ASSOCIATION, INC. 2007-11-09 |
 QUALITY CHOICE 77326104 3440519 Live/Registered |
CHAIN DRUG MARKETING ASSOCIATION, INC. 2007-11-09 |
 QUALITY CHOICE 76571887 2964690 Live/Registered |
CHAIN DRUG MARKETING ASSOCIATION, INC. 2004-01-26 |
 QUALITY CHOICE 74561878 2144847 Live/Registered |
CHAIN DRUG MARKETING ASSOCIATION, INC. 1994-08-17 |
 QUALITY CHOICE 74035467 not registered Dead/Abandoned |
Manhattan Life Insurance Co., The 1990-03-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.