EVERYDAY ESSENTIAL TRAVEL SALVES 4PC KIT- arnica montana, rosa moschata, achillea millefolium, matricaria recutita, salvia officinalis, comfrey, st johns wort, stellaria media, plantago major, commiphora myrrha kit
Everyday Essential Travel Salves 4pc Kit by
Drug Labeling and Warnings
Everyday Essential Travel Salves 4pc Kit by is a Homeopathic medication manufactured, distributed, or labeled by Sierra Sage Herbs LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Purpose Section
-
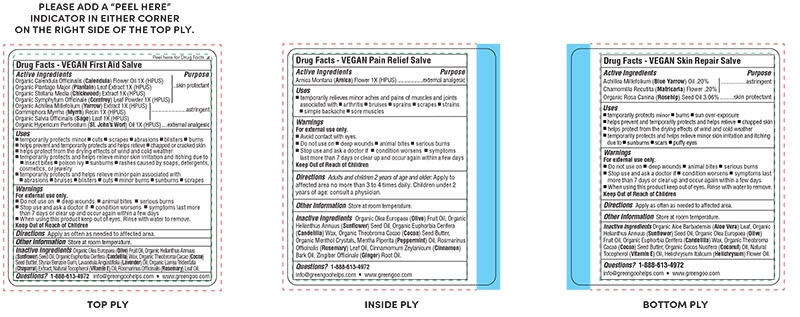
Active Ingredients Section
Organic Calendula Officinalis (Calendula) Flower Oil 1X (HPUS)
Organic Plantago Major (Plantain) Leaf Extract 1X (HPUS)
Organic Stellaria Media (Chickweed) Extract 1X (HPUS)
Organic Symphytum Officinale (Comfrey) Leaf Powder 1X (HPUS)
OrganicAchilleaMillefolium(Yarrow)Extract1X(HPUS)
Commiphora Myrrha (Myrrh) Resin 1X (HPUS)
Organic Salvia Officinalis (Sage) Leaf 1X (HPUS)
Organic Hypericum Perforatum (St. John’s Wort) Oil 1X (HPUS)
-
Indications & Usage
temporarily protects minor cuts scrapes abrasions bruises blisters burns
▪ temporarily protects and helps relieve chapped or cracked skin
▪ helps protect from the drying effects of wind and cold weather
▪ temporarily protects and helps relieve minor skin irritations and itching due to insect bites poison ivy sunburns
rashes caused by soaps, detergents, cosmetics, or jewelry
▪ temporarily protects and helps relieves minor pain associated with abrasions bruises blisters cuts minor burns sunburns
scrapes
- Keep out of Reach of Children
- Warnings Section
- Storage & Handling Section
- Dosage & Administration Section
-
Inactive Ingredients Section
Organic Olea Europaea (Olive) Fruit Oil, Organic Helianthus Annuus (Sunflower) Seed Oil, Organic Euphorbia Cerifera (Candelilla) Wax, Organic Theobroma Cacao (Cocoa) Seed Butter, Styrax Benzoin Gum, Lavandula Angustifolia (Lavender) Oil, Organic Larrea Tridentata (Chaparral) Extract, Tocopherol (Vitamin E), Rosmarinus Officinalis (Rosemary) Leaf Oil.
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
EVERYDAY ESSENTIAL TRAVEL SALVES 4PC KIT
arnica montana, rosa moschata, achillea millefolium, matricaria recutita, salvia officinalis, comfrey, st johns wort, stellaria media, plantago major, commiphora myrrha kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70994-112 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70994-112-12 1 in 1 BOX; Type 0: Not a Combination Product 03/10/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 4 in 3 Part 2 4 in 2 Part 3 4 in 4 Part 4 4 Part 1 of 4 SKIN REPAIR VEGAN STICK .15OZ
rosa moschata, achillea millefolium, matricaria recutita salveProduct Information Item Code (Source) NDC: 70994-120 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROSA MOSCHATA OIL (UNII: J99W255AWF) (ROSA MOSCHATA OIL - UNII:J99W255AWF) ROSA MOSCHATA OIL 1 [hp_X] in 100 g ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 1 [hp_X] in 100 g MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 1 [hp_X] in 100 g Inactive Ingredients Ingredient Name Strength HELICHRYSUM ITALICUM FLOWER OIL (UNII: O97ZV7726K) COCONUT OIL (UNII: Q9L0O73W7L) ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) ALOE VERA LEAF (UNII: ZY81Z83H0X) OLIVE OIL (UNII: 6UYK2W1W1E) THEOBROMA CACAO WHOLE (UNII: EB048G1S9J) SUNFLOWER OIL (UNII: 3W1JG795YI) CANDELILLA WAX (UNII: WL0328HX19) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/10/2022 Part 2 of 4 DRY SKIN CARE VEGAN STICK .15OZ
chamomile, calendula officinalis, achillea millefolium, trifolium pratense, sambucus nigra salveProduct Information Item Code (Source) NDC: 70994-117 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) (TRIFOLIUM PRATENSE FLOWER - UNII:4JS0838828) TRIFOLIUM PRATENSE FLOWER 1 [hp_X] in 100 g CHAMOMILE (UNII: FGL3685T2X) (CHAMOMILE - UNII:FGL3685T2X) CHAMOMILE 1 [hp_X] in 100 g ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 1 [hp_X] in 100 g CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) (CALENDULA OFFICINALIS FLOWER - UNII:P0M7O4Y7YD) CALENDULA OFFICINALIS FLOWER 1 [hp_X] in 100 g SAMBUCUS NIGRA FLOWER (UNII: 07V4DX094T) (SAMBUCUS NIGRA FLOWER - UNII:07V4DX094T) SAMBUCUS NIGRA FLOWER 1 [hp_X] in 100 g Inactive Ingredients Ingredient Name Strength LAVENDER OIL (UNII: ZBP1YXW0H8) OLIVE OIL (UNII: 6UYK2W1W1E) JOJOBA OIL (UNII: 724GKU717M) SUNFLOWER OIL (UNII: 3W1JG795YI) CANDELILLA WAX (UNII: WL0328HX19) THEOBROMA CACAO WHOLE (UNII: EB048G1S9J) ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/10/2022 Part 3 of 4 FIRST AID VEGAN STICK .15OZ
achillea millefolium, salvia officinalis, comfrey, st johns wort, stellaria media, calendula officinalis, plantago major, commiphora myrrha salveProduct Information Item Code (Source) NDC: 70994-118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STELLARIA MEDIA (UNII: 2H03479QVR) (STELLARIA MEDIA - UNII:2H03479QVR) STELLARIA MEDIA 1 [hp_X] in 100 g ACHILLEA MILLEFOLIUM FLOWER (UNII: YQR8R0SQEA) (ACHILLEA MILLEFOLIUM FLOWER - UNII:YQR8R0SQEA) ACHILLEA MILLEFOLIUM FLOWER 1 [hp_X] in 100 g SALVIA OFFICINALIS WHOLE (UNII: M9C36LC10E) (SALVIA OFFICINALIS WHOLE - UNII:M9C36LC10E) SALVIA OFFICINALIS WHOLE 1 [hp_X] in 100 g COMFREY LEAF (UNII: DG4F8T839X) (COMFREY LEAF - UNII:DG4F8T839X) COMFREY LEAF 1 [hp_X] in 100 g ST. JOHN'S WORT (UNII: UFH8805FKA) (ST. JOHN'S WORT - UNII:UFH8805FKA) ST. JOHN'S WORT 1 [hp_X] in 100 g CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) (CALENDULA OFFICINALIS FLOWER - UNII:P0M7O4Y7YD) CALENDULA OFFICINALIS FLOWER 1 [hp_X] in 100 g COMMIPHORA MYRRHA WHOLE (UNII: UU81N77RI7) (COMMIPHORA MYRRHA WHOLE - UNII:UU81N77RI7) COMMIPHORA MYRRHA WHOLE 1 [hp_X] in 100 g PLANTAGO MAJOR (UNII: W2469WNO6U) (PLANTAGO MAJOR - UNII:W2469WNO6U) PLANTAGO MAJOR 1 [hp_X] in 100 g Inactive Ingredients Ingredient Name Strength OLIVE OIL (UNII: 6UYK2W1W1E) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) SUNFLOWER OIL (UNII: 3W1JG795YI) CANDELILLA WAX (UNII: WL0328HX19) LARREA TRIDENTATA LEAF (UNII: PK0TXD049P) STYRAX BENZOIN RESIN (UNII: FE663Z8IRO) THEOBROMA CACAO WHOLE (UNII: EB048G1S9J) ROSEMARY OIL (UNII: 8LGU7VM393) LAVENDER OIL (UNII: ZBP1YXW0H8) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/10/2022 Part 4 of 4 PAIN RELIEF VEGAN STICK .15OZ
arnica montana salveProduct Information Item Code (Source) NDC: 70994-119 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) (ARNICA MONTANA FLOWER - UNII:OZ0E5Y15PZ) ARNICA MONTANA FLOWER 1 [hp_X] in 100 g Inactive Ingredients Ingredient Name Strength CINNAMON OIL (UNII: E5GY4I6YCZ) MENTHOL (UNII: L7T10EIP3A) MENTHA PIPERITA (UNII: 79M2M2UDA9) SUNFLOWER OIL (UNII: 3W1JG795YI) CANDELILLA WAX (UNII: WL0328HX19) THEOBROMA CACAO WHOLE (UNII: EB048G1S9J) GINGER OIL (UNII: SAS9Z1SVUK) ROSEMARY OIL (UNII: 8LGU7VM393) OLIVE OIL (UNII: 6UYK2W1W1E) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/10/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/10/2022 Labeler - Sierra Sage Herbs LLC (006970452) Registrant - Sierra Sage Herbs LLC (006970452)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.