estradiol by Zydus Pharmaceuticals USA Inc. / ZYDUS NOVELTECH INC, USA / Zydus Lifesciences Limited ESTRADIOL patch
estradiol by
Drug Labeling and Warnings
estradiol by is a Prescription medication manufactured, distributed, or labeled by Zydus Pharmaceuticals USA Inc., ZYDUS NOVELTECH INC, USA, Zydus Lifesciences Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ESTRADIOL TRANSDERMAL SYSTEM safely and effectively. See full prescribing information for ESTRADIOL TRANSDERMAL SYSTEM.
ESTRADIOL transdermal system
Initial U.S. Approval: 1975WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, PROBABLE DEMENTIA and BREAST CANCER
See full prescribing information for complete boxed warning.
Estrogen-Alone Therapy
- There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens (5.2)
- The Women's Health Initiative (WHI) estrogen-alone substudy reported increased risks of stroke and deep vein thrombosis (DVT) (5.1)
- The WHI Memory Study (WHIMS) estrogen-alone ancillary study of WHI reported an increased risk of probable dementia in postmenopausal women 65 years of age and older (5.3)
- Do not use estrogen-alone therapy for the prevention of cardiovascular disease or dementia (5.1, 5.3)
Estrogen Plus Progestin Therapy
- The WHI estrogen plus progestin substudy reported increased risks of stroke, DVT, pulmonary embolism (PE), and myocardial infarction (MI) (5.1)
- The WHI estrogen plus progestin substudy reported increased risks of invasive breast cancer (5.2)
- The WHIMS estrogen plus progestin ancillary study of WHI reported an increased risk of probable dementia in postmenopausal women 65 years of age and older (5.3)
- Do not use estrogen plus progestogen therapy for the prevention of cardiovascular disease or dementia (5.1, 5.3)
INDICATIONS AND USAGE
Estradiol Transdermal System, USP is an estrogen indicated for:
- Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause (1.1)
- Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause (1.2)
Limitations of Use
When prescribing solely for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause, first consider the use of topical vaginal products.
- Treatment of Hypoestrogenism due to Hypogonadism, Castration or Primary Ovarian Failure (1.3)
- Prevention of Postmenopausal Osteoporosis (1.4)
Limitations of Use
When prescribing solely for the prevention of postmenopausal osteoporosis, first consider the use of non-estrogen medications.
Consider estrogen therapy only for women at significant risk of osteoporosis.
DOSAGE AND ADMINISTRATION
- Start therapy with Estradiol Transdermal System 0.025 mg per day applied to the skin once-weekly. Dosage adjustment should be guided by the clinical response (2.1)
- Place Estradiol Transdermal System on a clean, dry area of the lower abdomen (below the umbilicus) or upper quadrant of the buttock. Do not apply Estradiol Transdermal System to the breasts (2.5)
DOSAGE FORMS AND STRENGTHS
- Transdermal system 0.025 mg per day, 0.0375 mg per day, 0.05 mg per day, 0.06 mg per day, 0.075 mg per day and 0.1 mg per day (3)
CONTRAINDICATIONS
- Undiagnosed abnormal genital bleeding (4, 5.2)
- Breast cancer or a history of breast cancer (4, 5.2)
- Estrogen-dependent neoplasia (4, 5.2)
- Active DVT, PE or a history of these conditions (4, 5.1)
- Active arterial thromboembolic disease (for example, stroke or MI), or a history of these conditions (4, 5.1)
- Known anaphylactic reaction or angioedema, or hypersensitivity to Estradiol Transdermal System (4)
- Hepatic impairment or disease (4, 5.10)
- Protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions (≥10 percent) with Estradiol Transdermal System are breast pain, upper respiratory tract infections, headaches, abdominal pain, pain, and edema. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Inducers and/or inhibitors of CYP3A4 may affect estrogen drug metabolism and decrease or increase the estrogen plasma concentration. (7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, PROBABLE DEMENTIA and BREAST CANCER
1 INDICATIONS AND USAGE
1.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause
1.2 Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause
1.3 Treatment of Hypoestrogenism due to Hypogonadism, Castration, or Primary Ovarian Failure
1.4 Prevention of Postmenopausal Osteoporosis
2 DOSAGE AND ADMINISTRATION
2.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause
2.2 Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause
2.3 Treatment of Hypoestrogenism due to Hypogonadism, Castration, or Primary Ovarian Failure
2.4 Prevention of Postmenopausal Osteoporosis
2.5 Application of the Estradiol Transdermal System
2.6 Removal of the Estradiol Transdermal System
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Disorders
5.2 Malignant Neoplasms
5.3 Probable Dementia
5.4 Gallbladder Disease
5.5 Hypercalcemia
5.6 Visual Abnormalities
5.7 Addition of a Progestogen When a Woman Has Not Had a Hysterectomy
5.8 Elevated Blood Pressure
5.9 Exacerbation of Hypertriglyceridemia
5.10 Hepatic Impairment and/or Past History of Cholestatic Jaundice
5.11 Exacerbation of Hypothyroidism
5.12 Fluid Retention
5.13 Hypocalcemia
5.14 Exacerbation of Endometriosis
5.15 Hereditary Angioedema
5.16 Exacerbation of Other Conditions
5.17 Laboratory Tests
5.18 Drug-Laboratory Test Interactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Effects on Vasomotor Symptoms in Postmenopausal Women
14.2 Effects on Bone Mineral Density in Postmenopausal Women
14.3 Women's Health Initiative Studies
14.4 Women's Health Initiative Memory Study
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, PROBABLE DEMENTIA and BREAST CANCER
Estrogen-Alone Therapy
Endometrial Cancer
There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens. Adding a progestogen to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer. Perform adequate diagnostic measures, including directed or random endometrial sampling when indicated to rule out malignancy in postmenopausal women with undiagnosed, persistent or recurring abnormal genital bleeding [see Warnings and Precautions (5.2)].
Cardiovascular Disorders and Probable Dementia
The Women's Health Initiative (WHI) estrogen-alone substudy reported increased risks of stroke and deep vein thrombosis (DVT) in postmenopausal women (50 to 79 years of age) during 7.1 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg]-alone, relative to placebo [see Warnings and Precautions (5.1), and Clinical Studies (14.3)].
The WHI Memory Study (WHIMS) estrogen-alone ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age and older during 5.2 years of treatment with daily CE (0.625 mg)-alone, relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see Warnings and Precautions (5.3), Use in Specific Populations (8.5), and Clinical Studies (14.4)].
Do not use estrogen-alone therapy for the prevention of cardiovascular disease or dementia [see Warnings and Precautions (5.1, 5.3), and Clinical Studies (14.3, 14.4)].
Only daily oral 0.625 mg CE was studied in the estrogen-alone substudy of the WHI. Therefore, the relevance of the WHI findings regarding adverse cardiovascular events and dementia to lower CE doses, other routes of administration, or other estrogen-alone products is not known. Without such data, it is not possible to definitively exclude these risks or determine the extent of these risks for other products. Discuss with your patient the benefits and risks of estrogen-alone therapy, taking into account her individual risk profile.
Prescribe estrogens with or without progestogens at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Estrogen Plus Progestin Therapy
Cardiovascular Disorders and Probable Dementia
The WHI estrogen plus progestin substudy reported increased risks of DVT, pulmonary embolism (PE), stroke and myocardial infarction (MI) in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral CE (0.625 mg) combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo [see Warnings and Precautions (5.1), and Clinical Studies (14.3)].
The WHIMS estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age and older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see Warnings and Precautions (5.3), Use in Specific Populations (8.5), and Clinical Studies (14.4)].
Do not use estrogen plus progestogen therapy for the prevention of cardiovascular disease or dementia [see Warnings and Precautions (5.1, 5.3), and Clinical Studies (14.3, 14.4)].
Breast Cancer
The WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer [see Warnings and Precautions (5.2), and Clinical Studies (14.3)].
Only daily oral 0.625 mg CE and 2.5 mg MPA were studied in the estrogen plus progestin substudy of the WHI. Therefore, the relevance of the WHI findings regarding adverse cardiovascular events, dementia and breast cancer to lower CE plus other MPA doses, other routes of administration, or other estrogen plus progestogen products is not known. Without such data, it is not possible to definitively exclude these risks or determine the extent of these risks for other products. Discuss with your patient the benefits and risks of estrogen plus progestogen therapy, taking into account her individual risk profile.
Prescribe estrogens with or without progestogens at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
-
1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Generally, when estrogen is prescribed for a postmenopausal woman with a uterus, consider addition of a progestogen to reduce the risk of endometrial cancer. Generally, a woman without a uterus does not need to use a progestogen in addition to her estrogen therapy. In some cases, however, hysterectomized women who have a history of endometriosis may need a progestogen [see Warnings and Precautions (5.2, 5.14)].
Use estrogen-alone, or in combination with a progestogen at the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Reevaluate postmenopausal women periodically as clinically appropriate to determine if treatment is still necessary.
2.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause
Start therapy with Estradiol Transdermal System 0.025 mg per day applied to the skin once weekly. Make dosage adjustments based on the clinical response. Attempt to taper or discontinue Estradiol Transdermal System at 3 to 6 month intervals.
2.2 Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause
Start therapy with Estradiol Transdermal System 0.025 mg per day applied to the skin once weekly. Make dosage adjustments based on the clinical response. Attempt to taper or discontinue Estradiol Transdermal System at 3 to 6 month intervals.
2.3 Treatment of Hypoestrogenism due to Hypogonadism, Castration, or Primary Ovarian Failure
Start therapy with 0.025 mg per day applied to the skin once weekly. Make dose adjustment based on the clinical response.
2.4 Prevention of Postmenopausal Osteoporosis
Start therapy with Estradiol Transdermal System 0.025 mg per day applied to the skin once weekly.
2.5 Application of the Estradiol Transdermal System
- Place the adhesive side of Estradiol Transdermal System on a clean, dry area of the lower abdomen or the upper quadrant of the buttock.
- Do not apply Estradiol Transdermal System to or near the breasts.
- Rotate the sites of application, with an interval of at least 1-week allowed between applications to the same site.
- Select an area that is not oily, damaged, or irritated. Avoid the waistline, since tight clothing may rub the transdermal system off.
- Avoid application to areas where sitting would dislodge Estradiol Transdermal System.
- Apply Estradiol Transdermal System immediately after opening the pouch and removing the protective liner.
- Press Estradiol Transdermal System firmly in place with the fingers for at least 10 seconds, making sure there is good contact, especially around the edges.
- If the system lifts, apply pressure to maintain adhesion.
- In the event that a system falls off, reapply it to a different location. If the old system cannot be reapplied, apply a new system for the remainder of the 7-day dosing interval.
- Wear only one system at any one time during the 7-day dosing interval.
- Swimming, bathing, or using a sauna while using Estradiol Transdermal System has not been studied, and these activities may decrease the adhesion of the system and the delivery of estradiol.
2.6 Removal of the Estradiol Transdermal System
- Remove Estradiol Transdermal System carefully and slowly to avoid irritation of the skin.
- If any adhesive remains on the skin after removal of Estradiol Transdermal System, allow the area to dry for 15 minutes and then gently rub the area with an oil-based cream or lotion to remove the adhesive residue.
- Used patches still contain some active hormones. Carefully fold each patch in half so that it sticks to itself before throwing it away.
-
3 DOSAGE FORMS AND STRENGTHS
- Estradiol Transdermal System, 0.025 mg/day — each 7.5 cm2system contains 1.888 mg of estradiol USP
- Estradiol Transdermal System, 0.0375 mg/day — each 11.25 cm2system contains 2.832 mg of estradiol USP
- Estradiol Transdermal System, 0.05 mg/day — each 15 cm2system contains 3.777 mg of estradiol USP
- Estradiol Transdermal System, 0.06 mg/day — each 18 cm2system contains 4.532 mg of estradiol USP
- Estradiol Transdermal System, 0.075 mg/day — each 22.5 cm2system contains 5.665 mg of estradiol USP
- Estradiol Transdermal System, 0.1 mg/day — each 30 cm2system contains 7.553 mg of estradiol USP
-
4 CONTRAINDICATIONS
Estradiol Transdermal System is contraindicated in women with any of the following conditions:
- Undiagnosed abnormal genital bleeding [see Warnings and Precautions (5.2)]
- Breast cancer or history of breast cancer [see Warnings and Precautions (5.2)]
- Estrogen-dependent neoplasia [see Warnings and Precautions (5.2)]
- Active DVT, PE, or a history of these conditions [see Warnings and Precautions (5.1)]
- Active arterial thromboembolic disease (for example, stroke or MI), or a history of these conditions [see Warnings and Precautions (5.1)]
- Known anaphylactic reaction, or angioedema, or hypersensitivity to Estradiol Transdermal System
- Hepatic impairment or disease
- Protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Disorders
Increased risks of stroke and DVT are reported with estrogen-alone therapy. Increased risks of PE, DVT, stroke and MI are reported with estrogen plus progestin therapy. Immediately discontinue estrogen with or without progestogen therapy if any of these occur or are suspected.
Manage appropriately any risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus).
Stroke
The WHI estrogen-alone substudy reported a statistically significant increased risk of stroke in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 strokes per 10,000 women-years, respectively). The increase in risk was demonstrated in year 1 and persisted [see Clinical Studies (14.3)]. Immediately discontinue estrogen-alone therapy if a stroke occurs or is suspected.
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
The WHI estrogen plus progestin substudy reported a statistically significant increased risk of stroke in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 strokes per 10,000 women years, respectively) [see Clinical Studies (14.3)]. The increase in risk was demonstrated after the first year and persisted.1 Immediately discontinue estrogen plus progestogen therapy if a stroke occurs or is suspected.
Coronary Heart Disease
The WHI estrogen-alone substudy reported no overall effect on coronary heart disease (CHD) events (defined as nonfatal MI, silent MI, or CHD death) in women receiving estrogen-alone compared to placebo2 [see Clinical Studies (14.3)].
Subgroup analyses of women 50 to 59 years of age, who were less than 10 years since menopause, suggest a reduction (not statistically significant) of CHD events in those women receiving daily CE (0.625 mg)-alone compared to placebo (8 versus 16 per 10,000 women-years).1
The WHI estrogen plus progestin substudy reported an increased risk (not statistically significant) of CHD events in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women-years).1 An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 [see Clinical Studies (14.3)].
In postmenopausal women with documented heart disease (n = 2,763), average 66.7 years of age, in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in HERS, HERS II, and overall.
Venous Thromboembolism
In the WHI estrogen-alone substudy, the risk of VTE (DVT and PE) was increased for women receiving daily CE (0.625 mg)-alone compared to placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women-years). The increase in VTE risk was demonstrated during the first 2 years3 [see Clinical Studies (14.3)]. Immediately discontinue estrogen-alone therapy if a VTE occurs or is suspected.
The WHI estrogen plus progestin substudy reported a statistically significant 2-fold greater rate of VTE in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women-years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted4 [see Clinical Studies (14.3)]. Immediately discontinue estrogen plus progestogen therapy if a VTE occurs or is suspected.
If feasible, discontinue estrogens at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
5.2 Malignant Neoplasms
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among unopposed estrogen users is about 2 to 12 times greater than in non-users, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use, with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestogen therapy is important. Perform adequate diagnostic measures, including directed or random endometrial sampling when indicated, to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding with unknown etiology.
There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestogen to estrogen therapy in postmenopausal women has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
Breast Cancer
The WHI substudy of daily CE (0.625 mg)-alone provided information about breast cancer in estrogen-alone users. In the WHI estrogen-alone substudy, after an average follow-up of 7.1 years, daily CE (0.625 mg)-alone was not associated with an increased risk of invasive breast cancer [relative risk (RR) 0.80]5 [see Clinical Studies (14.3)].
After a mean follow-up of 5.6 years, the WHI substudy of daily CE (0.625 mg) plus MPA (2.5 mg) reported an increased risk of invasive breast cancer in women who took daily CE plus MPA compared to placebo.
In this substudy, prior use of estrogen-alone or estrogen plus progestin therapy was reported by 26 percent of the women. The relative risk of invasive breast cancer was 1.24, and the absolute risk was 41 versus 33 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported prior use of hormone therapy, the relative risk of invasive breast cancer was 1.86, and the absolute risk was 46 versus 25 cases per 10,000 women-years for CE plus MPA compared with placebo.6 Among women who reported no prior use of hormone therapy, the relative risk of invasive breast cancer was 1.09, and the absolute risk was 40 versus 36 cases per 10,000 women-years for CE plus MPA compared with placebo. In the same substudy, invasive breast cancers were larger, were more likely to be node positive, and were diagnosed at a more advanced stage in the CE (0.625 mg) plus MPA (2.5 mg) group compared with the placebo group. Metastatic disease was rare, with no apparent difference between the two groups. Other prognostic factors, such as histologic subtype, grade and hormone receptor status did not differ between the groups6 [see Clinical Studies (14.3)].
Consistent with the WHI clinical trial, observational studies have also reported an increased risk of breast cancer with estrogen plus progestin therapy, and a smaller increase in the risk for breast cancer with estrogen-alone therapy, after several years of use. One large meta-analysis of prospective cohort studies reported increased risks that were dependent upon duration of use and could last up to >10 years after discontinuation of estrogen plus progestin therapy and estrogenalonetherapy. Extension of the WHI trials also demonstrated increased breast cancer risk associated with estrogen plus progestin therapy. Observational studies also suggest that the risk of breast cancer was greater, and became apparent earlier, with estrogen plus progestin therapy as compared to estrogen-alone therapy. These studies have not generally found significant variation in the risk of breast cancer among different estrogen plus progestin combinations, doses, or routes of administration.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation. All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
Ovarian Cancer
The CE plus MPA substudy of WHI reported that estrogen plus progestin increased the risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for CE plus MPA versus placebo was 1.58 (95 percent CI, 0.77-3.24), but it was not statistically significant. The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years] vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27 to 1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
5.3 Probable Dementia
In the WHI Memory Study (WHIMS) estrogen-alone ancillary study, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo.
After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83-2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years8[see Use in Specific Populations (8.5), and Clinical Studies (14.4)].
In the WHIMS estrogen plus progestin ancillary study, a population of 4,532 postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21-3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years8 [see Use in Specific Populations (8.5), and Clinical Studies (14.4)].
When data from the two populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19-2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women8[see Use in Specific Populations (8.5), and Clinical Studies (14.4)].
5.4 Gallbladder Disease
A 2-to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
5.5 Hypercalcemia
Estrogen administration may lead to severe hypercalcemia in women with breast cancer and bone metastases. Discontinue estrogens, including Estradiol Transdermal System if hypercalcemia occurs, and take appropriate measures to reduce the serum calcium level.
5.6 Visual Abnormalities
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue Estradiol Transdermal System pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. Permanently discontinue estrogens, including Estradiol Transdermal System, if examination reveals papilledema or retinal vascular lesions.
5.7 Addition of a Progestogen When a Woman Has Not Had a Hysterectomy
Studies of the addition of a progestogen for 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
There are, however, possible risks that may be associated with the use of progestogens with estrogens compared to estrogen-alone regimens. These include an increased risk of breast cancer.
5.8 Elevated Blood Pressure
In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogens on blood pressure was not seen.
5.9 Exacerbation of Hypertriglyceridemia
In women with pre-existing hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis. Discontinue Estradiol Transdermal System if pancreatitis occurs.
5.10 Hepatic Impairment and/or Past History of Cholestatic Jaundice
Estrogens may be poorly metabolized in women with hepatic impairment. Exercise caution in any woman with a history of cholestatic jaundice associated with past estrogen use or with pregnancy. In the case of recurrence of cholestatic jaundice, discontinue Estradiol Transdermal System.
5.11 Exacerbation of Hypothyroidism
Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Women with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T4 and T3 serum concentrations in the normal range. Women dependent on thyroid hormone replacement therapy who are also receiving estrogens may require increased doses of their thyroid replacement therapy. Monitor thyroid function in these women during treatment with Estradiol Transdermal System to maintain their free thyroid hormone levels in an acceptable range.
5.12 Fluid Retention
Estrogens may cause some degree of fluid retention. Monitor any woman with a condition(s) that might predispose her to fluid retention, such as a cardiac or renal impairment. Discontinue estrogen-alone therapy, including Estradiol Transdermal System, with evidence of medically concerning fluid retention.
5.13 Hypocalcemia
Estrogen-induced hypocalcemia may occur in women with hypoparathyroidism. Consider whether the benefits of estrogen therapy, including Estradiol Transdermal System, outweigh the risks in such women.
5.14 Exacerbation of Endometriosis
A few cases of malignant transformation of residual endometrial implants have been reported in women treated posthysterectomy with estrogen-alone therapy. Consider the addition of progestogen therapy for women known to have residual endometriosis post-hysterectomy.
5.15 Hereditary Angioedema
Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema. Consider whether the benefits of estrogen therapy, including Estradiol Transdermal System, outweigh the risks in such women.
5.16 Exacerbation of Other Conditions
Estrogen therapy, including Estradiol Transdermal System, may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine, porphyria, systemic lupus erythematosus, and hepatic hemangiomas. Consider whether the benefits of estrogen therapy outweigh the risks in such women.
5.17 Laboratory Tests
Serum follicle stimulating hormone (FSH) and estradiol levels have not been shown to be useful in the management of postmenopausal women with moderate to severe vasomotor symptoms and moderate to severe symptoms of vulvar and vaginal atrophy.
Laboratory parameters may be useful in guiding Estradiol Transdermal System dosage for the treatment of hypoestrogenism due to hypogonadism, castration and primary ovarian failure.
5.18 Drug-Laboratory Test Interactions
- Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex, and beta-thromboglobulin; decreased levels of antifactor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
- Increased TBG levels leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4levels (by column or by radioimmunoassay) or T3levels by radioimmunoassay. T3resin uptake is decreased, reflecting the elevated TBG. Free T4and free T3concentrations are unaltered. Women on thyroid replacement therapy may require higher doses of thyroid hormone.
- Other binding proteins may be elevated in serum, for example, corticosteroid binding globulin (CBG), sex hormone-binding globulin (SHBG), leading to increased total circulating corticosteroids and sex steroids, respectively. Free hormone concentrations, such as testosterone and estradiol, may be decreased. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-l-antitrypsin, ceruloplasmin).
- Increased plasma high-density lipoprotein (HDL) and HDL2cholesterol subfraction concentrations, reduced low-density lipoprotein (LDL) cholesterol concentration, and increased triglyceride levels.
- Impaired glucose tolerance.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Cardiovascular Disorders [see Boxed Warning, and Warnings and Precautions (5.1)]
- Malignant Neoplasms [see Boxed Warning, and Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect pooled data from 5 clinical trials of Estradiol Transdermal System. A total of 614 women were exposed to Estradiol Transdermal System for 3 months (193 women at 0.025 mg per day, 201 women at 0.05 mg per day, 194 women at 0.1 mg per day) in randomized, double-blind trials of clinical efficacy versus placebo and versus active comparator. All women were postmenopausal, had a serum estradiol level of less than 20 pg/mL, and a minimum of five moderate to severe hot flushes per week or a minimum of 15 hot flushes per week of any severity at baseline. Included in this table are an additional 25 postmenopausal hysterectomized women exposed to Estradiol Transdermal System 0.025 mg per day for 6 to 24 months (N=16 at 24 months) in a randomized, double-blind, placebo-controlled study of Estradiol Transdermal System for the prevention of osteoporosis.
Table 1 Treatment-Emergent Adverse Reactions Reported at a Frequency of ≥5 Percent and More Frequent in Women Receiving Estradiol Transdermal System a) Adverse reactions occurring at rate of ≥5 percent in Estradiol Transdermal System trials of clinical efficacy versus placebo and versus active comparator; and trial of Estradiol Transdermal System versus placebo for the prevention of osteoporosis
b) Adverse reactions occurring at rate of ≥5 percent in Estradiol Transdermal System trials of clinical efficacy versus placebo and versus active comparator
c) Adverse reactions occurring in placebo group in Estradiol Transdermal System trial of clinical efficacy versus placebo
Estradiol Transdermal System
Body System
Adverse Reactions
0.025 mg/daya
(N=219)
0.05 mg/dayb
(N=201)
0.1 mg/dayb
(N=194)
Placeboc
(N=72)
Body as a Whole
21%
39%
37%
29%
Headache
5%
18%
13%
10%
Pain
1%
8%
11%
7%
Back Pain
4%
8%
9%
6%
Edema
0.5%
13%
10%
6%
Digestive System
9%
21%
29%
18%
Abdominal Pain
0%
11%
16%
8%
Nausea
1%
5%
6%
3%
Flatulence
1%
3%
7%
1%
Musculoskeletal System
7%
9%
11%
4%
Arthralgia
1%
5%
5%
3%
Nervous System
13%
10%
11%
1%
Depression
1%
5%
8%
0%
Urogenital System
12%
18%
41%
11%
Breast Pain
5%
8%
29%
4%
Leukorrhea
1%
6%
7%
1%
Respiratory System
15%
26%
29%
14%
URTI
6%
17%
17%
8%
Pharyngitis
0.5%
3%
7%
3%
Sinusitis
4%
4%
5%
3%
Rhinitis
2%
4%
6%
1%
Skin and Appendages
19%
12%
12%
15%
Pruritus
0.5%
6%
3%
6%
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Estradiol Transdermal System. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Genitourinary System
Changes in bleeding pattern, pelvic pain
Breast
Breast cancer, breast pain, breast tenderness
Cardiovascular
Changes in blood pressure, palpitations, hot flashes
Gastrointestinal
Vomiting, abdominal pain, abdominal distension, nausea
Skin
Alopecia, hyperhidrosis, night sweats, urticaria, rash
Eyes
Visual disturbances, contact lens intolerance,
Central Nervous System
Depression, migraine, paresthesia, dizziness, anxiety, irritability, mood swings, nervousness, insomnia, headache
Miscellaneous
Fatigue, menopausal symptoms, weight increase, application site reaction, anaphylactic reactions
-
7 DRUG INTERACTIONS
In vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism. Inducers of CYP3A4 such as St. John's wort (hypericum perforatum) preparations, phenobarbital, carbamazepine, and rifampin may reduce plasma concentrations of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. Inhibitors of CYP3A4 such as erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir and grapefruit juice may increase plasma concentrations of estrogens and may result in adverse reactions.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Estradiol Transdermal System is not indicated for use in pregnancy. There are no data with the use of Estradiol Transdermal System in pregnant women; however, epidemiologic studies and meta-analyses have not found an increased risk of genital or nongenital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to combined hormonal contraceptives (estrogens and progestins) before conception or during early pregnancy.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Estrogens are present in human milk and can reduce milk production in breast-feeding women. This reduction can occur at any time but is less likely to occur once breast-feeding is well-established. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Estradiol Transdermal System and any potential adverse effects on the breastfed child from Estradiol Transdermal System or from the underlying maternal condition.
8.4 Pediatric Use
In general, Estradiol Transdermal System is not indicated for use in pediatric patients. Clinical studies have not been conducted in the pediatric population.
If estrogen is administered to patients whose bone growth is not complete, periodic monitoring of bone metabolism and effects on epiphyseal centers is recommended during estrogen administration.
8.5 Geriatric Use
There have not been sufficient numbers of geriatric women involved in clinical studies utilizing Estradiol Transdermal System to determine whether those over 65 years of age differ from younger subjects in their response to Estradiol Transdermal System.
The Women's Health Initiative Studies
In the WHI estrogen-alone substudy (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age [see Clinical Studies (14.3)].
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age [see Clinical Studies (14.3)].
The Women's Health Initiative Memory Study
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen-alone or estrogen plus progestin when compared to placebo [see Warnings and Precautions (5.3), and Clinical Studies (14.4)].
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women8[see Warnings and Precautions (5.3), and Clinical Studies (14.4)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Estradiol Transdermal System, USP is designed to release estradiol continuously upon application to intact skin. Six (7.5, 11.25, 15, 18, 22.5 and 30 cm2) systems are available to provide nominal in vivo delivery of 0.025, 0.0375, 0.05, 0.06, 0.075 or 0.1 mg respectively of estradiol per day. The period of use is 7 days. Each system has a contact surface area of either 7.5, 11.25, 15, 18, 22.5 or 30 cm2, and contains 1.888, 2.832, 3.777, 4.532, 5.665 or 7.553 mg of estradiol USP respectively. The composition of the systems per unit area is identical.
Estradiol USP is a white or creamy white, small crystals or crystalline powder, odorless, stable in air and hygroscopic. Estradiol USP is chemically described as estra-1,3,5(10)-triene-3, 17ß-diol. It has an empirical formula of C18H24O2 and molecular weight of 272.38. The structural formula is:
The Estradiol Transdermal System, USP comprises three layers. Proceeding from the visible surface toward the surface attached to the skin, these layers are:
- A translucent polyethylene film.
- An acrylate adhesive matrix containing estradiol USP.
- A protective liner of silicone coated polyester film is attached to the adhesive surface and must be removed before the system can be used.
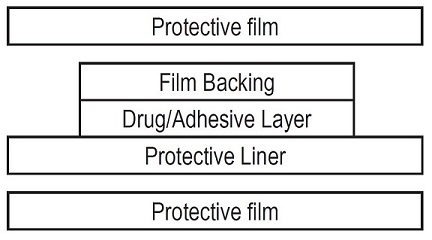
Estradiol Transdermal System, USP is packaged with additional pieces of protective film above and below the system within each pouch. These are discarded at the time of use.
The active component of the system is estradiol USP. The remaining components of the system (acrylic adhesive, colloidal silicon dioxide, ethyl oleate, glyceryl monolaurate, isopropyl myristate, povidone and polyethylene backing) are pharmacologically inactive.
FDA approved drug release test specifications differ from USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, which is secreted by the adrenal cortex, to estrone in the peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, two estrogen receptors have been identified. These vary in proportion from tissue to tissue.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and FSH, through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.
12.2 Pharmacodynamics
Generally, a serum estrogen concentration does not predict an individual woman's therapeutic response to Estradiol Transdermal System nor her risk for adverse outcomes. Likewise, exposure comparisons across different estrogen products to infer efficacy or safety for the individual woman may not be valid.
12.3 Pharmacokinetics
Transdermal administration of Estradiol Transdermal System produces mean serum concentrations of estradiol comparable to those produced by premenopausal women in the early follicular phase of the ovulatory cycle. The pharmacokinetics of estradiol following application of the Estradiol Transdermal System were investigated in 197 healthy postmenopausal women in six studies. In five of the studies, the Estradiol Transdermal System was applied to the abdomen and in a sixth study, application to the buttocks and abdomen were compared.
The Estradiol Transdermal System continuously releases estradiol which is transported across intact skin leading to sustained circulating levels of estradiol during a 7-day treatment period. The systemic availability of estradiol after transdermal administration is about 20 times higher than that after oral administration. This difference is due to the absence of first pass metabolism when estradiol is given by the transdermal route.
In a bioavailability study, the Estradiol Transdermal System, 0.025 mg per day was studied with the Estradiol Transdermal System, 0.05 mg per day as reference. The mean estradiol levels in serum from the two sizes are shown in Figure 1.
Figure 1: Mean Serum 17ß-Estradiol Concentrations versus Time Profile following Application of a 0.025 mg per day Transdermal System and Application of a 0.05 mg per day Estradiol Transdermal System
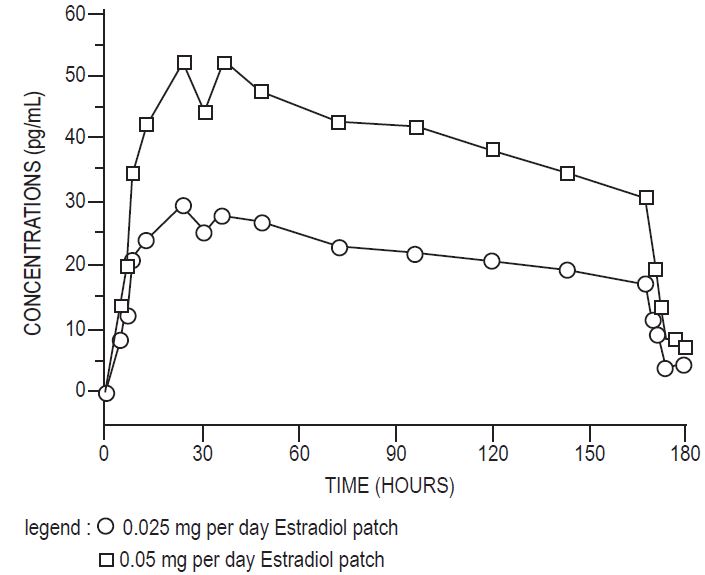
Dose proportionality was demonstrated for the Estradiol Transdermal System 0.025 mg per day as compared to the Estradiol Transdermal System 0.05 mg per day in a 2-week crossover study with a 1-week washout period between the two-transdermal systems in 24 postmenopausal women.
Dose proportionality was also demonstrated for the Estradiol Transdermal System (0.05 mg per day and 0.1 mg per day) in a 1-week study conducted in 54 postmenopausal women. The mean steady state levels (Cavg) of the estradiol during the application of Estradiol Transdermal System 0.1 mg per day and 0.05 mg per day on the abdomen were about 80 and 40 pg/mL, respectively.
In a 3-week multiple application study in 24 postmenopausal women, the 0.1 mg per day Estradiol Transdermal System produced average peak estradiol concentrations (Cmax) of approximately 100 pg/mL. Trough values at the end of each wear interval (Cmin) were approximately 35 pg/mL. Nearly identical serum curves were seen each week, indicating little or no accumulation of estradiol in the body. Serum estrone peak and trough levels were 60 and 40 pg/mL, respectively.
In a single dose, randomized, crossover study conducted to compare the effect of site of application, 38 postmenopausal women wore a single Estradiol Transdermal System 0.1 mg per day for 1 week on the abdomen and buttocks. The estradiol serum concentration profiles are shown in Figure 2. Values of Cmax and Cavg were, respectively, 25 percent and 17 percent higher with the buttock application than with the abdomen application.
Figure 2: Observed Mean (± SE) Estradiol Serum Concentrations for a One Week Application of the Estradiol Transdermal System (0.1 mg per day) to the Abdomen and Buttocks of 38 Postmenopausal Women
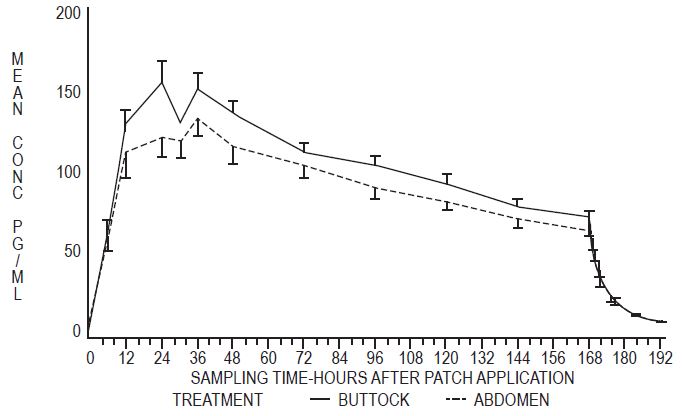
Table 2 provides a summary of estradiol pharmacokinetic parameters determined during evaluation of the Estradiol Transdermal System.
Table 2
Pharmacokinetic Summary (Mean Estradiol Values)
Estradiol Transdermal System Delivery Rate
Surface Area (cm2)
Application Site
No. of Subjects
Dosing
Cmax (pg/mL)
Cmin (pg/mL)
Cavg (pg/mL)
0.025
7.5
Abdomen
24
Single
32
17
22
0.05
15
Abdomen
102
Single
71
29
41
0.1
30
Abdomen
139
Single
147
60
87
0.1
30
Buttock
38
Single
174
71
106
The relative standard deviation of each pharmacokinetic parameter after application to the abdomen averaged 50 percent, which is indicative of the considerable intersubject variability associated with transdermal drug delivery. The relative standard deviation of each pharmacokinetic parameter after application to the buttock was lower than that after application to the abdomen (for example, for Cmax 39 percent versus 62 percent, and for Cavg 35 percent versus 48 percent).
Distribution
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estrogens circulate in the blood largely bound to SHBG and albumin.
Metabolism
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is a major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the intestine followed by reabsorption. In postmenopausal women, a significant proportion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
Excretion
Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates.
Adhesion
An open-label study of adhesion potentials of placebo transdermal systems that correspond to the 0.025 mg per day and 0.05 mg per day Estradiol Transdermal System was conducted in 112 healthy women of 45 to 75 years of age. Each woman applied both transdermal systems weekly, on the upper outer abdomen, for 3 consecutive weeks. It should be noted that lower abdomen and upper quadrant of the buttock are the approved sites of application for Estradiol Transdermal System.
The adhesion assessment was done visually on Days 2, 4, 5, 6, 7 of each week of transdermal system wear. A total of 1,654 adhesion observations were conducted for 333 transdermal systems of each size.
Of these observations, approximately 90 percent showed essentially no lift for both the 0.025 mg per day and 0.05 mg per day transdermal systems. Of the total number of transdermal systems applied, approximately 5 percent showed complete detachment for each size. Adhesion potentials of the 0.075 mg per day and 0.1 mg per day transdermal systems have not been studied.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Effects on Vasomotor Symptoms in Postmenopausal Women
A study of 214 women 25 to 74 years of age met the qualification criteria and were randomly assigned to one of the three treatment groups: 72 to the 0.05 mg estradiol patch, 70 to the 0.1 mg estradiol patch, and 72 to placebo. Potential participants were postmenopausal women in good general health who experienced vasomotor symptoms. Natural menopause patients had not menstruated for at least 12 months and surgical menopause patients had undergone bilateral oophorectomy at least 4 weeks before evaluation for study entry. In order to enter the 11-week treatment phase of the study, potential participants must have experienced a minimum of five moderate to severe hot flushes per week, or a minimum of 15 hot flushes of any severity per week, for 2 consecutive weeks. Women wore the patches in a cyclical fashion (three weeks on and one week off).
During treatment, all women used diaries to record the number and severity of hot flushes. Women were monitored by clinic visits at the end of weeks 1, 3, 7, and 11 and by telephone at the end of weeks 4, 5, 8, and 9.
Adequate data for the analysis of efficacy was available from 191 subjects. The results are presented as the mean ± SD number of flushes in each of the 3 treatment weeks of each 4-week cycle. In the 0.05 mg estradiol group, the mean weekly hot flush rate across all treatment cycles decreased from 46 ± 6.5 at baseline to 20 ± 3 (-67 percent). The 0.1 mg estradiol group had a decline in the mean weekly hot flush rate from 52 ± 4.4 at baseline to 16 ± 2.4 (-72 percent). In the placebo group, the mean weekly hot flush rate declined from 53 ± 4.5 at baseline to 46 ± 6.5 (-18.1 percent). Compared with placebo, the 0.05 mg and 0.1 mg estradiol groups showed a statistically significantly larger mean decrease in hot flushes across all treatment cycles (P<0.05). When the response to treatment was analyzed for each of the three cycles of therapy, similar statistically significant differences were observed between both estradiol treatment groups and the placebo group during all treatment cycles.
In a double-blind, placebo-controlled, randomized study of 187 women receiving Estradiol Transdermal System 0.025 mg per day or placebo continuously for up to three 28-day cycles, the Estradiol Transdermal System 0.025 mg per day dosage was shown to be statistically better than placebo at weeks 4 and 12 for relief of both the frequency and severity of moderate to severe vasomotor symptoms.
Table 3
Mean Change from Baseline in the Number of Moderate to Severe Vasomotor Symptoms Intent to Treat (ITT)
Treatment Group
Statistics
Week 4
Week 8
Week 12
E2 Transdermal System
N
82
84
68
Mean
-6.45
-7.69
-7.56
SD
4.65
4.76
4.64
Placebo
N
83
71
65
Mean
-5.11
-5.98
-5.98
SD
7.43
8.63
9.69
p-value
<0.002
<0.003
A second active-control trial of 193 randomized women was supportive of the placebo-controlled trial.
14.2 Effects on Bone Mineral Density in Postmenopausal Women
A two-year clinical trial enrolled a total of 175 healthy, hysterectomized, postmenopausal, non-osteoporotic (that is, lumbar spine bone mineral density >0.9 gm/cm2) women at 10 study centers in the United States. A total of 129 participating women were allocated to receive active treatment with 4 different doses of estradiol patches (0.025, 0.05, 0.06, 0.1 mg per day) and 46 participating women were allocated to receive placebo patches. Seventy-seven percent of the randomized women (100 on active drug and 34 on placebo) contributed data to the analysis of percent change of anterior-posterior (A-P) spine BMD, the primary efficacy variable (see Figure 3). A statistically significant overall treatment effect at each timepoint was noted, implying bone preservation for all active treatment groups at all timepoints, as opposed to bone loss for placebo at all timepoints.
Figure 3: Mean Percent Change from Baseline in Lumbar Spine (A-P View) Bone Mineral Density By Treatment and Time Last Observation Carried Forward
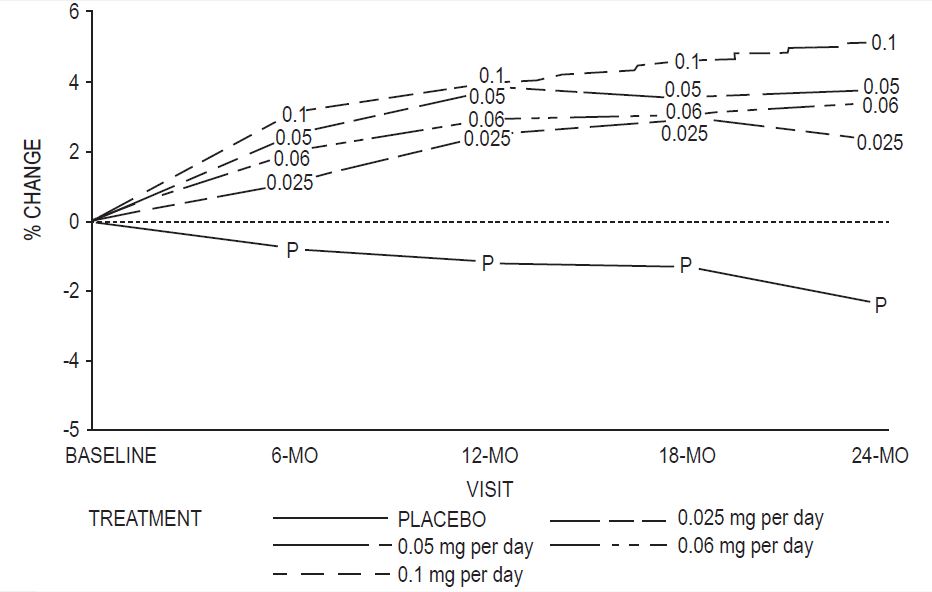
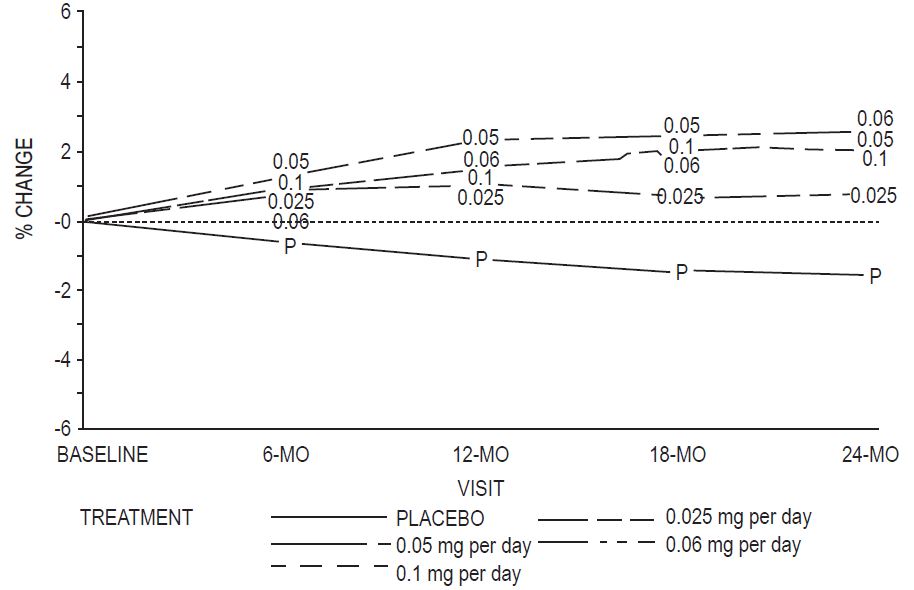
Percent change in BMD of the total hip (see Figure 4) was also statistically significantly different from placebo for all active treatment groups. This figure is based on 74 percent of the randomized women (95 on active drug and 34 on placebo).
Figure 4: Mean Percent Change from Baseline in Total Hip by Treatment and Time Last Observation Carried Forward
14.3 Women's Health Initiative Studies
The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of CHD (defined as nonfatal MI, silent MI and CHD death), with invasive breast cancer as the primary adverse outcome. A "global index" included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other causes. These substudies did not evaluate the effects of CE-alone or CE plus MPA on menopausal symptoms.
WHI Estrogen-Alone Substudy
The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risk and benefits of estrogen-alone in predetermined primary endpoints.
Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79: 75.3 percent White, 15.1 percent Black, 6.1 percent Hispanic, 3.6 percent Other) after an average follow-up of 7.1 years, are presented in Table 4.
Table 4
Relative and Absolute Risk Seen in the Estrogen-Alone Substudy of WHIa
a) Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi .
b) Nominal confidence intervals unadjusted for multiple looks and multiple comparisons.
c) Results are based on centrally adjudicated data for an average follow-up of 7.1 years.
d) Not included in "global index".
e) Results are based on an average follow-up of 6.8 years.
f) All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.
g) A subset of the events was combined in a "global index", defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture, or death due to other causes.
Event
Relative Risk CE vs. Placebo (95% nCIb)
CE
n = 5,310
Placebo
n = 5,429
Absolute Risk per 10,000
Women- years
CHD eventsc
0.95 (0.78-1.16)
54
57
Non-fatal MIc
0.91 (0.73-1.14)
40
43
CHD deathc
1.01 (0.71-1.43)
16
16
All strokesc
1.33 (1.05-1.68)
45
33
Ischemic strokec
1.55 (1.19-2.01)
38
25
Deep vein thrombosisc,d
1.47 (1.06-2.06)
23
15
Pulmonary embolismc
1.37 (0.9-2.07)
14
10
Invasive breast cancerc
0.80 (0.62-1.04)
28
34
Colorectal cancerc
1.08 (0.75-1.55)
17
16
Hip fracturec
0.65 (0.45-0.94)
12
19
Vertebral fracturesc,d
0.64 (0.44-0.93)
11
18
Lower arm/wrist fracturesc,d
0.58 (0.47-0.72)
35
59
Total fracturesc,d
0.71 (0.64-0.80)
144
197
Death due to causese,f
1.08 (0.88-1.32)
53
50
Overall mortalityc,d
1.04 (0.88-1.22)
79
75
Global Indexg
1.02 (0.92-1.13)
206
201
For those outcomes included in the WHI "global index" that reached statistical significance, the absolute excess risks per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures.9 The absolute excess risk of events included in the "global index" was a non-significant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years. See Table 4.
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant difference in the distribution of stroke subtype and severity, including fatal strokes, in women receiving estrogen-alone compared to placebo. Estrogen-alone increased the risk of ischemic stroke, and this excess risk was present in all subgroups of women examined.10 See Table 4.
Timing of initiation of estrogen-alone therapy relative to the start of menopause may affect the overall risk-benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age a non-significant trend toward reduced risk for CHD [hazard ratio (HR) 0.63 (95 percent CI, 0.36-1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46-1.11)].
WHI Estrogen Plus Progestin Substudy
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the "global index". The absolute excess risk of events included in the "global index" was 19 per 10,000 women-years.
For those outcomes included in the WHI "global index" that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women-years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reduction per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the CE plus MPA substudy, which included 16,608 women (average 63 years of age, range 50 to 79; 83.9 percent White, 6.5 percent Black, 5.4 percent Hispanic, 3.9 percent Other), are presented in Table 5 . These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
Table 5
Relative and Absolute Risk Seen in the Estrogen Plus Progestin Substudy of WHI at an Average of 5.6 Yearsa,b
a)Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi .
b) Results are based on centrally adjudicated data.
c) Nominal confidence intervals unadjusted for multiple looks and multiple comparisons.
d) Not included in "global index".
e) Includes metastatic and non-metastatic breast cancer, with the exception of in situ breast cancer.
f) All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.
g) A subset of the events was combined in a "global index", defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture, or death due to other causes.
Event
Relative Risk CE/MPA vs. placebo (95% nCIc)
CE/MPA
n = 8,506
Placebo
n = 8,102
Absolute Risk per 10,000
Women-years
CHD events
Non-fatal MI
CHD death
1.23 (0.99-1.53)
1.28 (1.00 -1.63)
1.10 (0.70-1.75)
41
31
8
34
25
8
All strokes
1.31 (1.03-1.68)
33
25
Ischemic stroke
1.44 (1.09-1.90)
26
18
Deep vein thrombosisd
1.95 (1.43-2.67)
26
13
Pulmonary embolism
2.13 (1.45-3.11)
18
8
Invasive breast cancere
1.24 (1.01-1.54)
41
33
Colorectal cancer
0.61 (0.42-0.87)
10
16
Endometrial cancerd
0.81 (0.48-1.36)
6
7
Cervical cancerd
1.44 (0.47-4.42)
2
1
Hip fracture
0.67 (0.47-0.96)
11
16
Vertebral fracturesd
0.65 (0.46-0.92)
11
17
Lower arm/wrist fracturesd
0.71 (0.59-0.85)
44
62
Total fracturesd
0.76 (0.69-0.83)
152
199
Overall mortalityf
1.00 (0.83-1.19)
52
52
Global Indexg
1.13 (1.02-1.25)
184
165
Timing of initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a non-significant trend toward reduced risk for overall mortality [HR 0.69 (95 percent CI, 0.44-1.07)].
14.4 Women's Health Initiative Memory Study
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age and older (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83-2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in the study included Alzheimer's disease (AD), vascular dementia (VaD) and mixed types (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women [see Warnings and Precautions (5.3), and Use in Specific Populations (8.5)].
The WHIMS estrogen plus progestin ancillary study enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years of age; and 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21-3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years. Probable dementia as defined in the study included AD, VaD and mixed types (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women [see Warnings and Precautions (5.3), and Use in Specific Populations (8.5)].
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19-2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women [see Warnings and Precautions (5.3), and Use in Specific Populations (8.5)].
-
15 REFERENCES
- Rossouw JE, et al. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause. JAMA. 2007;297:1465-1477.
- Hsia J, et al. Conjugated Equine Estrogens and Coronary Heart Disease. Arch Int Med. 2006;166:357-365.
- Curb JD, et al. Venous Thrombosis and Conjugated Equine Estrogen in Women Without a Uterus. Arch Int Med. 2006;166:772-780.
- Cushman M, et al. Estrogen Plus Progestin and Risk of Venous Thrombosis. JAMA. 2004;292:1573-1580.
- Stefanick ML, et al. Effects of Conjugated Equine Estrogens on Breast Cancer and Mammography Screening in Postmenopausal Women With Hysterectomy. JAMA. 2006;295:1647-1657.
- Chlebowski RT, et al. Influence of Estrogen Plus Progestin on Breast Cancer and Mammography in Healthy Postmenopausal Women. JAMA. 2003;289:3234-3253.
- Anderson GL, et al. Effects of Estrogen Plus Progestin on Gynecologic Cancers and Associated Diagnostic Procedures. JAMA. 2003;290:1739-1748.
- Shumaker SA, et al. Conjugated Equine Estrogens and Incidence of Probable Dementia and Mild Cognitive Impairment in Postmenopausal Women. JAMA. 2004;291:2947-2958.
- Jackson RD, et al. Effects of Conjugated Equine Estrogen on Risk of Fractures and BMD in Postmenopausal Women With Hysterectomy: Results From the Women's Health Initiative Randomized Trial. J Bone Miner Res. 2006;21:817-828.
- Hendrix SL, et al. Effects of Conjugated Equine Estrogen on Stroke in the Women's Health Initiative. Circulation. 2006;113:2425-2434.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Estradiol Transdermal System, USP, 0.025 mg / day — each 7.5 cm2 system contains 1.888 mg of estradiol USP
Individual Carton of 4 systems………………NDC: 68382-324-04
Estradiol Transdermal System, USP, 0.0375 mg / day — each 11.25 cm2 system contains 2.832 mg of estradiol USP
Individual Carton of 4 systems………………NDC: 68382-325-04
Estradiol Transdermal System, USP, 0.05 mg / day — each 15 cm2 system contains 3.777 mg of estradiol USP
Individual Carton of 4 systems………………NDC: 68382-326-04
Estradiol Transdermal System, USP, 0.06 mg / day — each 18 cm2 system contains 4.532 mg of estradiol USP
Individual Carton of 4 systems………………NDC: 68382-327-04
Estradiol Transdermal System, USP, 0.075 mg / day — each 22.5 cm2 system contains 5.665 mg of estradiol USP
Individual Carton of 4 systems………………NDC: 68382-328-04
Estradiol Transdermal System, USP, 0.1 mg / day — each 30 cm2 system contains 7.553 mg of estradiol USP
Individual Carton of 4 systems………………NDC: 68382-329-04
16.2 Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [See USP for Controlled Room Temperature].
Do not store unpouched. Do not store above 86°F (30°C). Apply immediately upon removal from the protective pouch.
Used transdermal systems still contain active hormone. To discard, fold the sticky side of the transdermal system together, place it in a sturdy child-proof container, and place this container in the trash. Used transdermal systems should not be flushed in the toilet.
-
17 PATIENT COUNSELING INFORMATION
Advise women to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
Vaginal Bleeding
Inform postmenopausal women to report any vaginal bleeding to their healthcare provider as soon as possible [see Warning and Precautions (5.2)].
Possible Serious Adverse Reactions with Estrogen-Alone Therapy
Inform postmenopausal women of possible serious adverse reactions of estrogen-alone therapy including Cardiovascular Disorders, Malignant Neoplasms, and Probable Dementia [see Warnings and Precautions (5.1, 5.2, 5.3)].
Possible Common Adverse Reactions with Estrogen-Alone Therapy
Inform postmenopausal women of possible less serious but common adverse reactions of estrogen-alone therapy such as headache, breast pain and tenderness, nausea and vomiting.
Manufactured by:
Zydus Lifesciences Ltd.
Ahmedabad, India
Distributed by:
Zydus Pharmaceuticals (USA) Inc.
Pennington, NJ 08534
Rev.: 03/24
-
Patient Information
Estradiol Transdermal System, USP
(es" tra dye' ol)
Rx only
Read this Patient Information before you start using Estradiol Transdermal System and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your menopausal symptoms or your treatment.
What is the most important information I should know about Estradiol Transdermal System (an estrogen hormone)?
Using estrogen-alone may increase your chance of getting cancer of the uterus (womb).
Report any unusual vaginal bleeding right away while you are using Estradiol Transdermal System. Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
Do not use estrogen-alone to prevent heart disease, heart attacks, strokes, or dementia (decline in brain function).
Using estrogen-alone may increase your chances of getting strokes or blood clots.
Using estrogen-alone may increase your chance of getting dementia, based on a study of women age 65 years of age and older.
Do not use estrogens with progestogens to prevent heart disease, heart attacks, strokes or dementia.
Using estrogens with progestogens may increase your chances of getting heart attacks, strokes, breast cancer, or blood clots.
Using estrogens with progestogens may increase your chance of getting dementia, based on a study of women age 65 years of age and older.
Only one estrogen-alone product and dose have been shown to increase your chances of getting strokes, blood clots, and dementia. Only one estrogen with progestogen product and dose have been shown to increase your chances of getting heart attacks, strokes, breast cancer, blood clots, and dementia.
Because other products and doses have not been studied in the same way, it is not known how the use of Estradiol Transdermal System will affect your chances of these conditions. You and your healthcare provider should talk regularly about whether you still need treatment with Estradiol Transdermal System.
What is Estradiol Transdermal System?
Estradiol Transdermal System is a prescription medicine patch (transdermal system) that contains estradiol (an estrogen hormone).
What is Estradiol Transdermal System used for?
The Estradiol Transdermal System is used after menopause to:
Reduce moderate to severe hot flashes
Estrogens are hormones made by a woman's ovaries. The ovaries normally stop making estrogens when a woman is between 45 and 55 years old. This drop in body estrogen levels causes the "change of life" or menopause (the end of monthly menstrual periods). Sometimes, both ovaries are removed during an operation before natural menopause takes place. The sudden drop in estrogen levels causes "surgical menopause."
When estrogen levels begin dropping, some women develop very uncomfortable symptoms, such as feelings of warmth in the face, neck, and chest, or sudden intense feelings of heat and sweating ("hot flashes" or "hot flushes"). In some women, the symptoms are mild, and they will not need to use estrogens. In other women, symptoms can be more severe.
Treat moderate to severe menopausal changes in and around the vagina
You and your healthcare provider should talk regularly about whether you still need treatment with Estradiol Transdermal System to control these problems. If you use Estradiol Transdermal System only to treat your menopausal changes in and around your vagina, talk with your healthcare provider about whether a topical vaginal product would be better for you.
Treat certain conditions in women before menopause if their ovaries do not produce enough estrogens naturally
Help reduce your chances of getting osteoporosis (thin weak bones)
Osteoporosis from menopause is a thinning of the bones that makes them weaker and easier to break. If you use Estradiol Transdermal System only to prevent osteoporosis due to menopause, talk with your healthcare provider about whether a different treatment or medicine without estrogens might be better for you.
You and your healthcare provider should talk regularly about whether you still need treatment with Estradiol Transdermal System.
Who should not use Estradiol Transdermal System?
Do not start using Estradiol Transdermal System if you:
have unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
have been diagnosed with a bleeding disorder
currently have or have had certain cancers
Estrogens may increase the chance of getting certain types of cancers, including cancer of the breast or uterus (womb). If you have or have had cancer, talk with your healthcare provider about whether you should use Estradiol Transdermal System.
had a stroke or heart attack
currently have or have had blood clots
currently have or have had liver problems
are allergic to Estradiol Transdermal System or any of the ingredients in it. See the list of ingredients in Estradiol Transdermal System at the end of this leaflet.
Before you use Estradiol Transdermal System, tell your healthcare provider about all of your medical conditions, including if you:
have any unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
have any other medical conditions that may become worse while you are using Estradiol Transdermal System
Your healthcare provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis, lupus, angioedema (swelling of face and tongue), or problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood.
are going to have surgery or will be on bed rest.
Your healthcare provider will let you know if you need to stop using Estradiol Transdermal System.
are pregnant or think you may be pregnant.
Estradiol Transdermal System is not for pregnant women.
are breastfeeding
The hormone in Estradiol Transdermal System can pass into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how Estradiol Transdermal System works. Estradiol Transdermal System may also affect how your other medicines work. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get new medicine.
How should I use Estradiol Transdermal System?
For detailed instructions, see the step-by-step instructions for using Estradiol Transdermal System at the end of this Patient Information.
Use Estradiol Transdermal System exactly as your healthcare provider tells you to use it.
Estradiol Transdermal System is for skin use only.
Change your Estradiol Transdermal System patch 1 time each week or every 7 days.
Apply your Estradiol Transdermal System patch to a clean, dry area on your lower abdomen or buttocks. This area must be clean, dry, and free of powder, oil or lotion for your patch to stick to your skin.
Apply your Estradiol Transdermal System patch to a different area of your abdomen or your buttocks each time. Do not use the same application site 2 times in the same week.
Do not apply Estradiol Transdermal System to your breasts.
If you forget to apply a new Estradiol Transdermal System patch, apply a new patch as soon as possible.
You and your healthcare provider should talk regularly (every 3 to 6 months) about the dose you are using and whether you still need treatment with Estradiol Transdermal System.
How to Change Estradiol Transdermal System
When changing Estradiol Transdermal Sysem, peel off the used patch slowly from the skin.
After removal of Estradiol Transdermal Sysem if any adhesive residue remains on your skin, allow the area to dry for 15 minutes. Then, gently rub the area with an oil-based cream or lotion to remove the adhesive from your skin.
Apply the new patch to a different area of your abdomen or buttocks. This area must be clean, dry, and free of powder, oil or lotion. Do not use the same site again for at least 1 week after removal of an old patch.
What are the possible side effects of Estradiol Transdermal System?
Side effects are grouped by how serious they are and how often they happen when you are treated.
Serious, but less common side effects include:
heart attack
stroke
blood clots
breast cancer
cancer of the lining of the uterus (womb)
cancer of the ovary
dementia
high or low blood calcium
gallbladder disease
visual abnormalities
high blood pressure
high levels of fat (triglyceride) in your blood
liver problems
changes in your thyroid hormone levels
fluid retention
cancer changes of endometriosis
enlargement of benign tumors of the uterus ("fibroids")
worsening of swelling of face and tongue (angioedema) in women with a history of angioedema
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- new breast lumps
- unusual vaginal bleeding
- changes in vision or speech
- sudden new severe headaches
- severe pains in your chest or legs with or without shortness of breath, weakness and
- fatigue
Common side effects of Estradiol Transdermal System include:
headache
breast tenderness or pain
irregular vaginal bleeding or spotting
stomach or abdominal cramps, bloating
nausea and vomiting
hair loss
fluid retention
vaginal yeast infection
redness and/or irritation at the patch placement site
These are not all the possible side effects of Estradiol Transdermal System. For more information, ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effects that bother you or do not go away.
You may report side effects to FDA at 1-800-FDA-1088. You may report side effects to Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779.
What can I do to lower my chances of a serious side effect with Estradiol Transdermal System?
- Talk with your healthcare provider regularly about whether you should continue using Estradiol Transdermal System.
- If you have a uterus, talk with your healthcare provider about whether the addition of a progestogen is right for you.
- In general, the addition of a progestogen is generally recommended for a woman with a uterus to reduce the chance of getting cancer of the uterus (womb).
- See your healthcare provider right away if you get vaginal bleeding while using Estradiol Transdermal System.
- Have a pelvic exam, breast exam and mammogram (breast X-ray) every year unless your healthcare provider tells you something else.
- If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram, you may need to have breast exams more often.
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have higher chances for getting heart disease. Ask your healthcare provider for ways to lower your chances of getting heart disease.
How should I store and throw away used Estradiol Transdermal System?
- Store Estradiol Transdermal System at 68°F to 77°F (20°C to 25°C).
- Do not store Estradiol Transdermal System patches outside of their pouches. Apply immediately upon removal from the protective pouch.
- Used patches still contain estrogen. To throw away the patch, fold the sticky side of the patch together, place it in a sturdy child-proof container, and place this container in the trash. Used patches should not be flushed in the toilet.
Keep Estradiol Transdermal System and all medicines out of the reach of children.
General information about the safe and effective use of Estradiol Transdermal System.
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use Estradiol Transdermal System for conditions for which it was not prescribed. Do not give Estradiol Transdermal System to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Estradiol Transdermal System that is written for health professionals.
Please address medical inquiries to, (MedicalAffairs@zydususa.com) Tel.: 1-877-993-8779.
What are the ingredients in Estradiol Transdermal System?
Active ingredient: estradiol
Inactive ingredient: acrylic adhesive, colloidal silicon dioxide, ethyl oleate, glyceryl monolaurate, isopropyl myristate, povidone and polyethylene backing.
INSTRUCTIONS FOR USE
Estradiol Transdermal System, USP (es" tra dye' ol)
Read this Patient Information before you start using Estradiol Transdermal System and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your menopausal symptoms or your treatment.
You will need the following supplies: See Figure A

Step 1: Pick the days you will change your Estradiol Transdermal System.
- You will need to change your patch 1 time each week or every 7 days.
Step 2. Remove the Estradiol Transdermal System patch from the pouch.
- Remove patch from its protective pouch by tearing at the notch (do not use scissors). See Figure B
- Do not remove your patch from the protective pouch until you are ready to apply it.
Step 3. Remove the adhesive liner. See Figure C
- You will see that Estradiol Transdermal System is a translucent rectangular shaped patch with round corners that is attached to a thick, hard-plastic adhesive liner. Estradiol Transdermal System is packaged with additional pieces of protective film above and below the system within each pouch. These are discarded at the time of use. See Figure C
- To apply your patch you must first remove the protective, clear plastic film that is attached to the clear thicker plastic backing. See Figure D
Step 4. Placing the patch on your skin.
- Apply the sticky side of the patch to 1 of the areas of skin shown below. See Figure E and Figure F
- Do not touch the sticky side of the patch with your fingers.
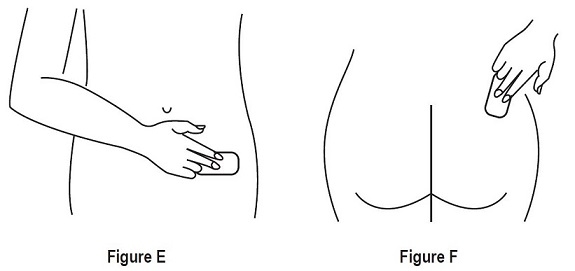
- Avoid the waistline, since clothing and belts may cause the patch to be rubbed off.
- Do not apply Estradiol Transdermal System to your breasts.
- Only apply Estradiol Transdermal System to skin that is clean, dry, and free of any powder, oil, or lotion.
- Do not apply the patch to injured, burned, or irritated skin, or areas with skin conditions (such as birth marks, tattoos, or that is very hairy).
Step 5. Press the patch firmly onto your skin
- Press the patch firmly in place with your fingers for at least 10 seconds
- Rub the edges of the patch to make sure that it will stick to your skin. (See Figure G)
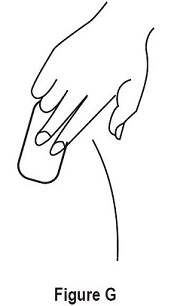
- Contact with water while you are swimming, using a sauna, bathing, or showering may cause the patch to fall off.
- If your patch falls off reapply it. If you cannot reapply the patch, apply a new patch to another area (See Figures E and F ), and continue to follow your original application schedule.
- If you stop using your Estradiol Transdermal System patch or forget to apply a new patch as scheduled, you may have spotting, or bleeding, and your symptoms may come back.
Step 6: Throwing away your used patch.
- When it is time to change your patch, remove the old patch before you apply a new patch.
- To throw away the patch, fold the sticky side of the patch together, place it in a sturdy childproof container, and place this container in the trash. Do not flush used patches in the toilet.
This Patient Information and Instructions for Use have been approved by the U.S Food and Drug Administration.
Manufactured by:
Zydus Lifesciences Ltd.
Ahmedabad, India
Distributed by:
Zydus Pharmaceuticals (USA) Inc.
Pennington, NJ 08534
Rev.: 05/22
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Estradiol Transdermal System, USP
0.025 mg/day
Continuous Delivery (Once-Weekly)
FOR TRANSDERMAL USE ONLY
Contains 4 transdermal systems
Rx only
zydus Pharmaceuticals USA
Estradiol Transdermal System, USP
0.0375 mg/day
Continuous Delivery (Once-Weekly)
FOR TRANSDERMAL USE ONLY
Contains 4 transdermal systems
Rx only
zydus Pharmaceuticals USA

Label
Estradiol Transdermal System, USP
0.05 mg/day
Continuous Delivery (Once-Weekly)
FOR TRANSDERMAL USE ONLY
Contains 4 transdermal systems
Rx only
zydus Pharmaceuticals USA
Estradiol Transdermal System, USP
0.06 mg/day
Continuous Delivery (Once-Weekly)
FOR TRANSDERMAL USE ONLY
Contains 4 transdermal systems
Rx only
zydus Pharmaceuticals USA
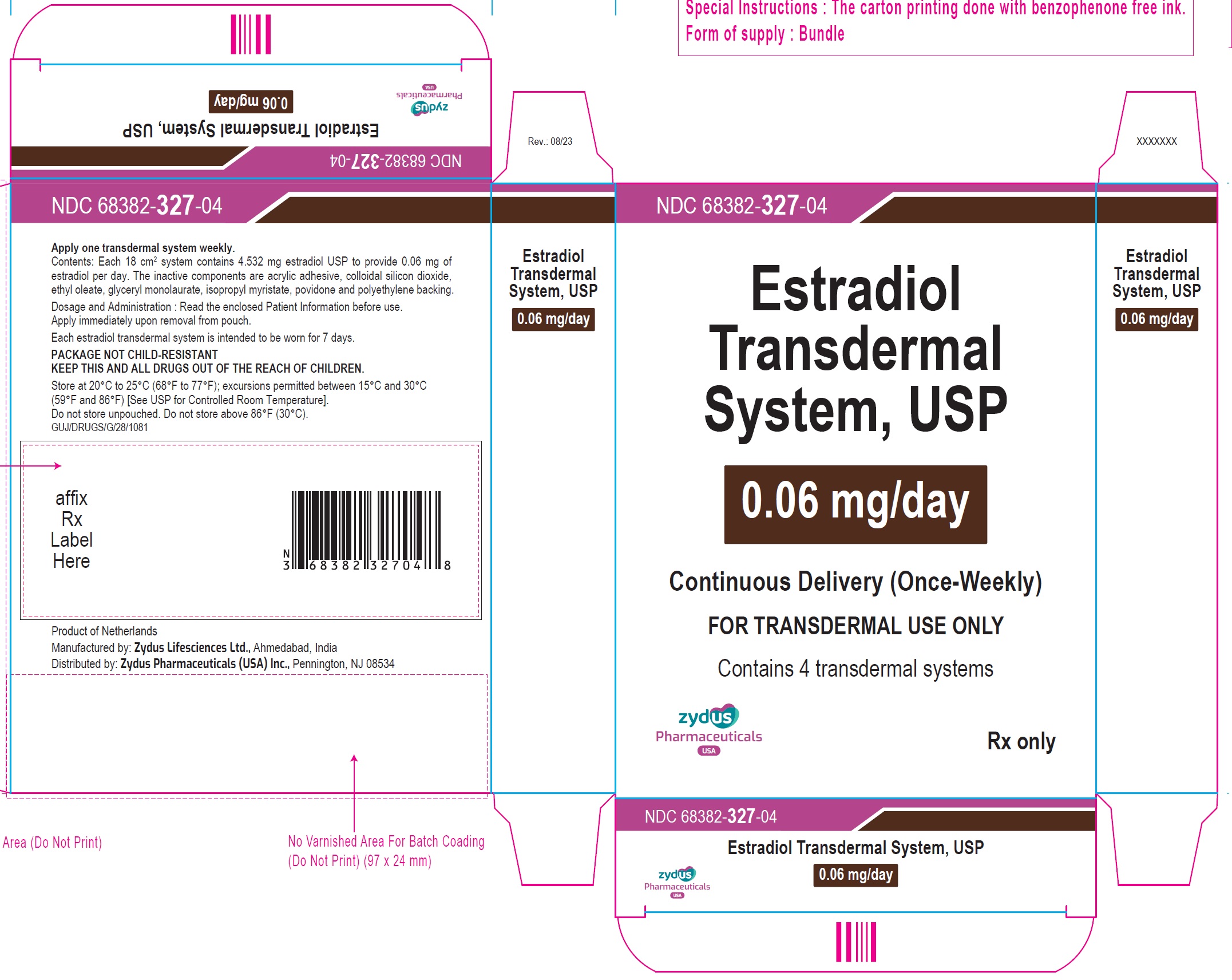
Estradiol Transdermal System, USP
0.075 mg/day
Continuous Delivery (Once-Weekly)
FOR TRANSDERMAL USE ONLY
Contains 4 transdermal systems
Rx only
zydus Pharmaceuticals USA

Estradiol Transdermal System, USP
0.1 mg/day
Continuous Delivery (Once-Weekly)
FOR TRANSDERMAL USE ONLY
Contains 4 transdermal systems
Rx only
zydus Pharmaceuticals USA
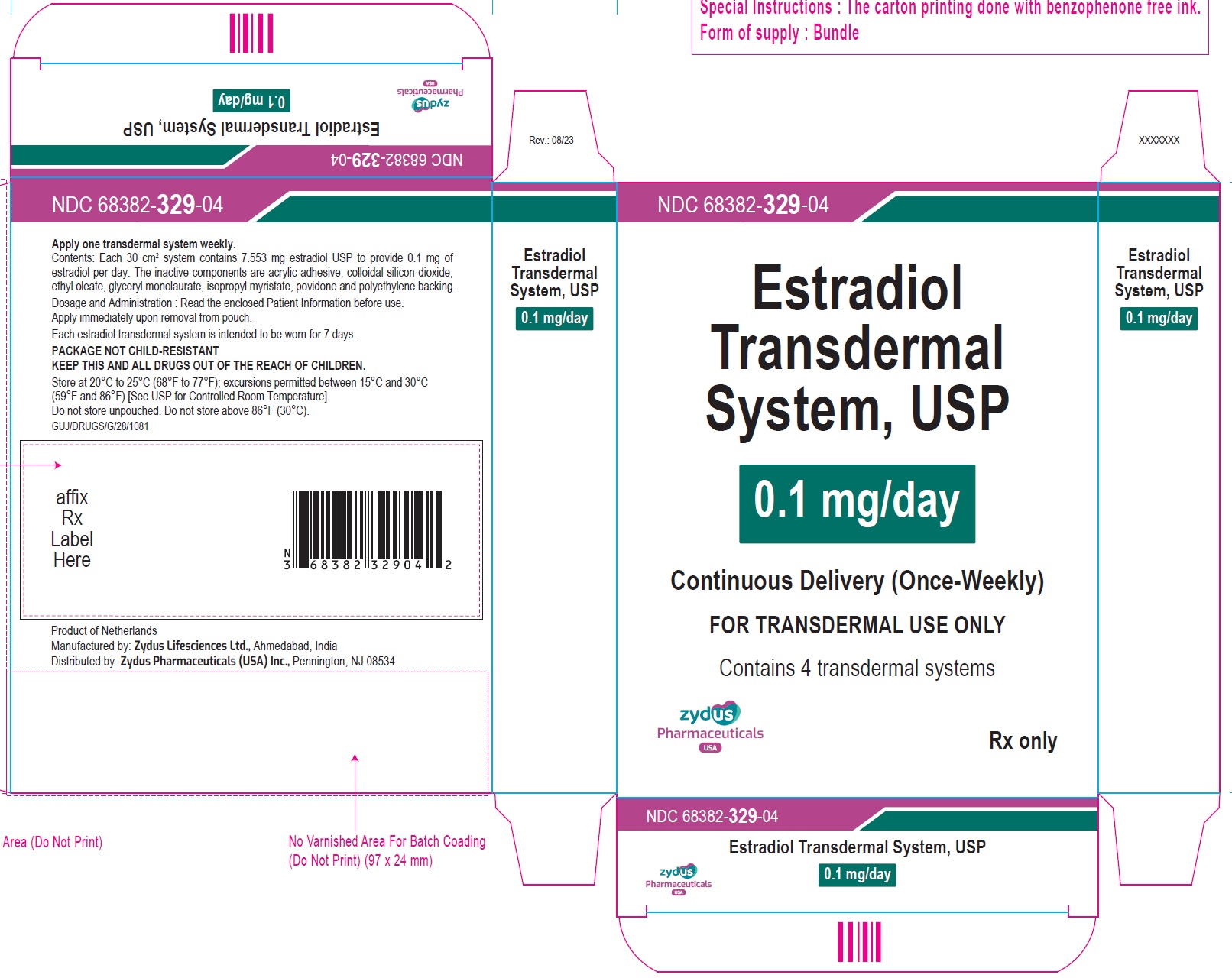
-
INGREDIENTS AND APPEARANCE
ESTRADIOL
estradiol patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-324 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 0.025 mg in 1 d Inactive Ingredients Ingredient Name Strength 2-ETHYLHEXYL ACRYLATE (UNII: HR49R9S6XG) 2-HYDROXYETHYL ACRYLATE (UNII: 25GT92NY0C) ETHYL OLEATE (UNII: Z2Z439864Y) GLYCERYL LAURATE (UNII: Y98611C087) GLYCIDYL METHACRYLATE (UNII: R8WN29J8VF) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) VINYL ACETATE (UNII: L9MK238N77) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-324-04 4 in 1 CARTON 11/02/2023 1 NDC: 68382-324-01 1 in 1 POUCH 1 7 d in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202985 11/02/2023 ESTRADIOL
estradiol patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-325 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 0.0375 mg in 1 d Inactive Ingredients Ingredient Name Strength 2-ETHYLHEXYL ACRYLATE (UNII: HR49R9S6XG) 2-HYDROXYETHYL ACRYLATE (UNII: 25GT92NY0C) ETHYL OLEATE (UNII: Z2Z439864Y) GLYCERYL LAURATE (UNII: Y98611C087) GLYCIDYL METHACRYLATE (UNII: R8WN29J8VF) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) VINYL ACETATE (UNII: L9MK238N77) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-325-04 4 in 1 CARTON 11/02/2023 1 NDC: 68382-325-01 1 in 1 POUCH 1 7 d in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202985 11/02/2023 ESTRADIOL
estradiol patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-326 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 0.05 mg in 1 d Inactive Ingredients Ingredient Name Strength 2-ETHYLHEXYL ACRYLATE (UNII: HR49R9S6XG) 2-HYDROXYETHYL ACRYLATE (UNII: 25GT92NY0C) ETHYL OLEATE (UNII: Z2Z439864Y) GLYCERYL LAURATE (UNII: Y98611C087) GLYCIDYL METHACRYLATE (UNII: R8WN29J8VF) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) VINYL ACETATE (UNII: L9MK238N77) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-326-04 4 in 1 CARTON 11/02/2023 1 NDC: 68382-326-01 1 in 1 POUCH 1 7 d in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202985 11/02/2023 ESTRADIOL
estradiol patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-327 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 0.06 mg in 1 d Inactive Ingredients Ingredient Name Strength 2-ETHYLHEXYL ACRYLATE (UNII: HR49R9S6XG) 2-HYDROXYETHYL ACRYLATE (UNII: 25GT92NY0C) ETHYL OLEATE (UNII: Z2Z439864Y) GLYCERYL LAURATE (UNII: Y98611C087) GLYCIDYL METHACRYLATE (UNII: R8WN29J8VF) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) VINYL ACETATE (UNII: L9MK238N77) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-327-04 4 in 1 CARTON 11/02/2023 1 NDC: 68382-327-01 1 in 1 POUCH 1 7 d in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202985 11/02/2023 ESTRADIOL
estradiol patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-328 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 0.075 mg in 1 d Inactive Ingredients Ingredient Name Strength 2-ETHYLHEXYL ACRYLATE (UNII: HR49R9S6XG) 2-HYDROXYETHYL ACRYLATE (UNII: 25GT92NY0C) ETHYL OLEATE (UNII: Z2Z439864Y) GLYCERYL LAURATE (UNII: Y98611C087) GLYCIDYL METHACRYLATE (UNII: R8WN29J8VF) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) VINYL ACETATE (UNII: L9MK238N77) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-328-04 4 in 1 CARTON 11/02/2023 1 NDC: 68382-328-01 1 in 1 POUCH 1 7 d in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202985 11/02/2023 ESTRADIOL
estradiol patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-329 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESTRADIOL (UNII: 4TI98Z838E) (ESTRADIOL - UNII:4TI98Z838E) ESTRADIOL 0.1 mg in 1 d Inactive Ingredients Ingredient Name Strength 2-ETHYLHEXYL ACRYLATE (UNII: HR49R9S6XG) 2-HYDROXYETHYL ACRYLATE (UNII: 25GT92NY0C) ETHYL OLEATE (UNII: Z2Z439864Y) GLYCERYL LAURATE (UNII: Y98611C087) GLYCIDYL METHACRYLATE (UNII: R8WN29J8VF) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) VINYL ACETATE (UNII: L9MK238N77) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-329-04 4 in 1 CARTON 11/02/2023 1 NDC: 68382-329-01 1 in 1 POUCH 1 7 d in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202985 11/02/2023 Labeler - Zydus Pharmaceuticals USA Inc. (156861945) Registrant - ZYDUS NOVELTECH INC, USA (801012530) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(68382-324, 68382-325, 68382-326, 68382-327, 68382-328, 68382-329) , MANUFACTURE(68382-324, 68382-325, 68382-326, 68382-327, 68382-328, 68382-329)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.