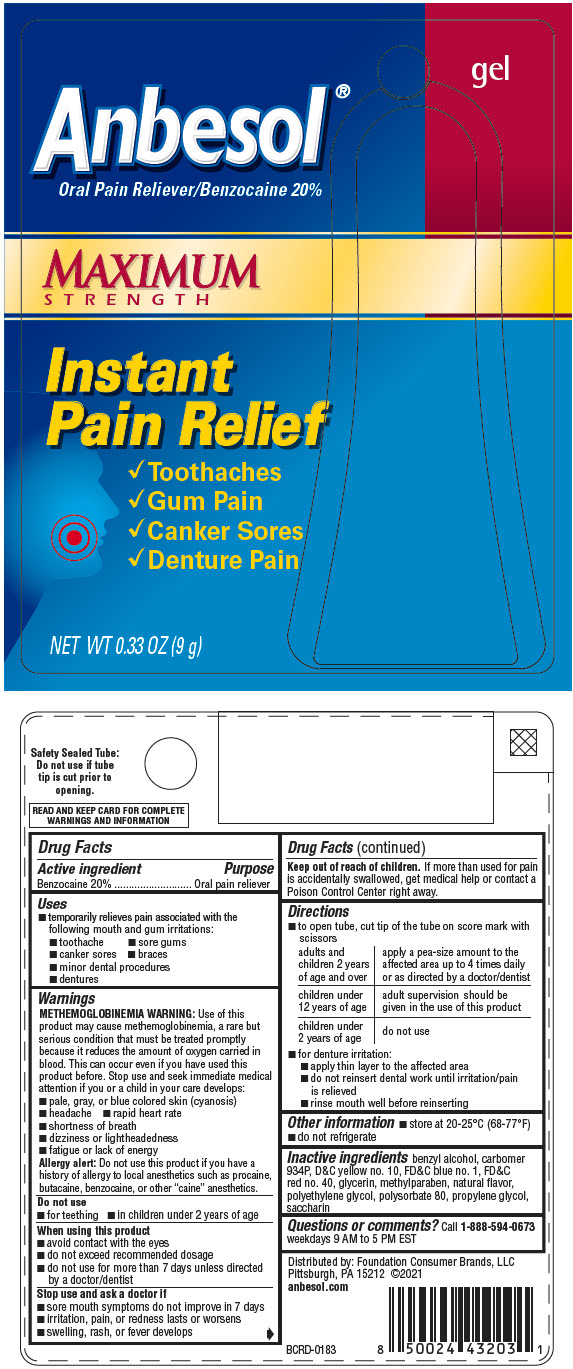
ANBESOL MAXIMUM STRENGTH- benzocaine gel
Anbesol by
Drug Labeling and Warnings
Anbesol by is a Otc medication manufactured, distributed, or labeled by Foundation Consumer Brands. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
METHEMOGLOBINEMIA WARNING
Use of this product may cause methemoglobinemia, a rare but serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert
Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "caine" anesthetics.
When using this product
- avoid contact with the eyes
- do not exceed recommended dosage
- do not use for more than 7 days unless directed by a doctor/dentist
-
Directions
- to open tube, cut tip of the tube on score mark with scissors
adults and children 2 years of age and over apply a pea-size amount to the affected area up to 4 times daily or as directed by a doctor/dentist children under 12 years of age adult supervision should be given in the use of this product children under 2 years of age do not use - for denture irritation:
- apply thin layer to the affected area
- do not reinsert dental work until irritation/pain is relieved
- rinse mouth well before reinserting
- to open tube, cut tip of the tube on score mark with scissors
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 9 g Tube Blister Pack
-
INGREDIENTS AND APPEARANCE
ANBESOL MAXIMUM STRENGTH
benzocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 80070-220 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL OR ALLYL SUCROSE CROSSLINKED) (UNII: K6MOM3T5YL) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SACCHARIN (UNII: FST467XS7D) Product Characteristics Color brown Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 80070-220-33 1 in 1 BLISTER PACK 09/15/2021 1 9 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 09/15/2021 Labeler - Foundation Consumer Brands (117603632)
Trademark Results [Anbesol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ANBESOL 73218985 1154463 Live/Registered |
AMERICAN HOME PRODUCTS CORPORATION 1979-06-08 |
 ANBESOL 71353283 0319723 Live/Registered |
LISS, MICHAEL 1934-06-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.