VONAFLEX- lidocaine lotion lotion
Vonaflex by
Drug Labeling and Warnings
Vonaflex by is a Otc medication manufactured, distributed, or labeled by Cymbiotics, Inc, Cymbiotics, Inc., Westwood Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Uses
- Directions
-
WARNINGS
For External use only.
Do not use:on wounds, raw surfaces or blistered areas, with a heating pad, or if allergic to product ingredients
When using this product:Avoid eye contact; do not use excessive amounts; do not exceed recommended dosage unless directed by doctor; do not bandage applied area.
Stop and ask doctor if:an allergic reaction occurs; condition worsens or does not improve within 7 days.
If pregnant or breast-feeding:consult with doctor before use.
if swallowed, get medical help or contact a Poison Control Center.
- Other Information:
-
INACTIVE INGREDIENT
Arnica Montanaflower extract, cetearyl alcohol, cetyl ester waxes, cyclopentasiloxane, disodium EDTA, ethoxydiglycol, fragrance, Helianthus annuus(sunflower) oil, Lavandula Angustifolio(lavender) oil, methylparaben, PEG40 hydrogenated caster oil, poly(acrylic acid) 2-propionic acid homopolymer, propylene glycol, propylparaben, tetrahydrocurcumin, tocopherol acetate, triethanolamine, water.
- Questions and Comments
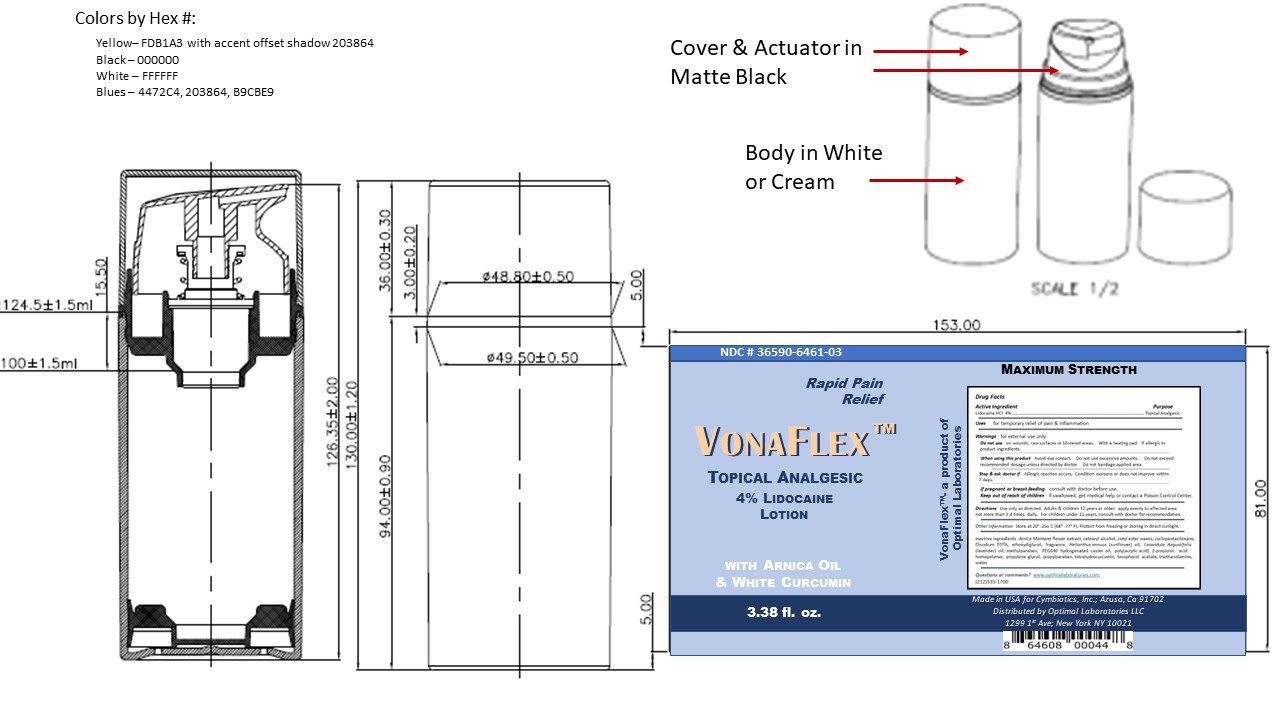
- Package Label and Principal Display Panel Vonaflex
-
INGREDIENTS AND APPEARANCE
VONAFLEX
lidocaine lotion lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 36590-6461 Route of Administration TOPICAL, CUTANEOUS, VAGINAL, RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) 0.2 g in 100 g EDETATE DISODIUM (UNII: 7FLD91C86K) 0.1 g in 100 g PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.02 g in 100 g WATER (UNII: 059QF0KO0R) 71.93 g in 100 g SUNFLOWER OIL (UNII: 3W1JG795YI) 0.75 g in 100 g CARBOMER 940 (UNII: 4Q93RCW27E) 0.55 g in 100 g LAVENDER OIL (UNII: ZBP1YXW0H8) 1 g in 100 g POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) 1 g in 100 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 4 g in 100 g DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) 5 g in 100 g CETYL ESTERS WAX (UNII: D072FFP9GU) 4 g in 100 g CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) 2 g in 100 g TETRAHYDRODIFERULOYLMETHANE (UNII: 00U0645U03) 2 g in 100 g TROLAMINE (UNII: 9O3K93S3TK) 1.5 g in 100 g .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1.2 g in 100 g CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) 0.4 g in 100 g FRAGRANCE LAVENDER ROSE ORC1004596 (UNII: 1XW43TV4PI) 0.1 g in 100 g ARNICA MONTANA FLOWER WATER (UNII: U7L2JP51PR) 0.25 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 36590-6461-1 50 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/25/2023 2 NDC: 36590-6461-2 75 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/25/2023 3 NDC: 36590-6461-3 100 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/26/2022 4 NDC: 36590-6461-4 150 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/26/2022 5 NDC: 36590-6461-5 200 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/25/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/25/2022 Labeler - Cymbiotics, Inc (781766709) Registrant - Westwood Laboratories, LLC (832280635) Establishment Name Address ID/FEI Business Operations Westwood Laboratories, LLC 832280635 manufacture(36590-6461) , label(36590-6461) , pack(36590-6461)
Trademark Results [Vonaflex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VONAFLEX 98382654 not registered Live/Pending |
Optimal Laboratories 2024-01-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.