PAIN RELIEF EXTRA STRENGTH- acetaminophen tablet, coated
Pain Relief by
Drug Labeling and Warnings
Pain Relief by is a Otc medication manufactured, distributed, or labeled by MARC GLASSMAN, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
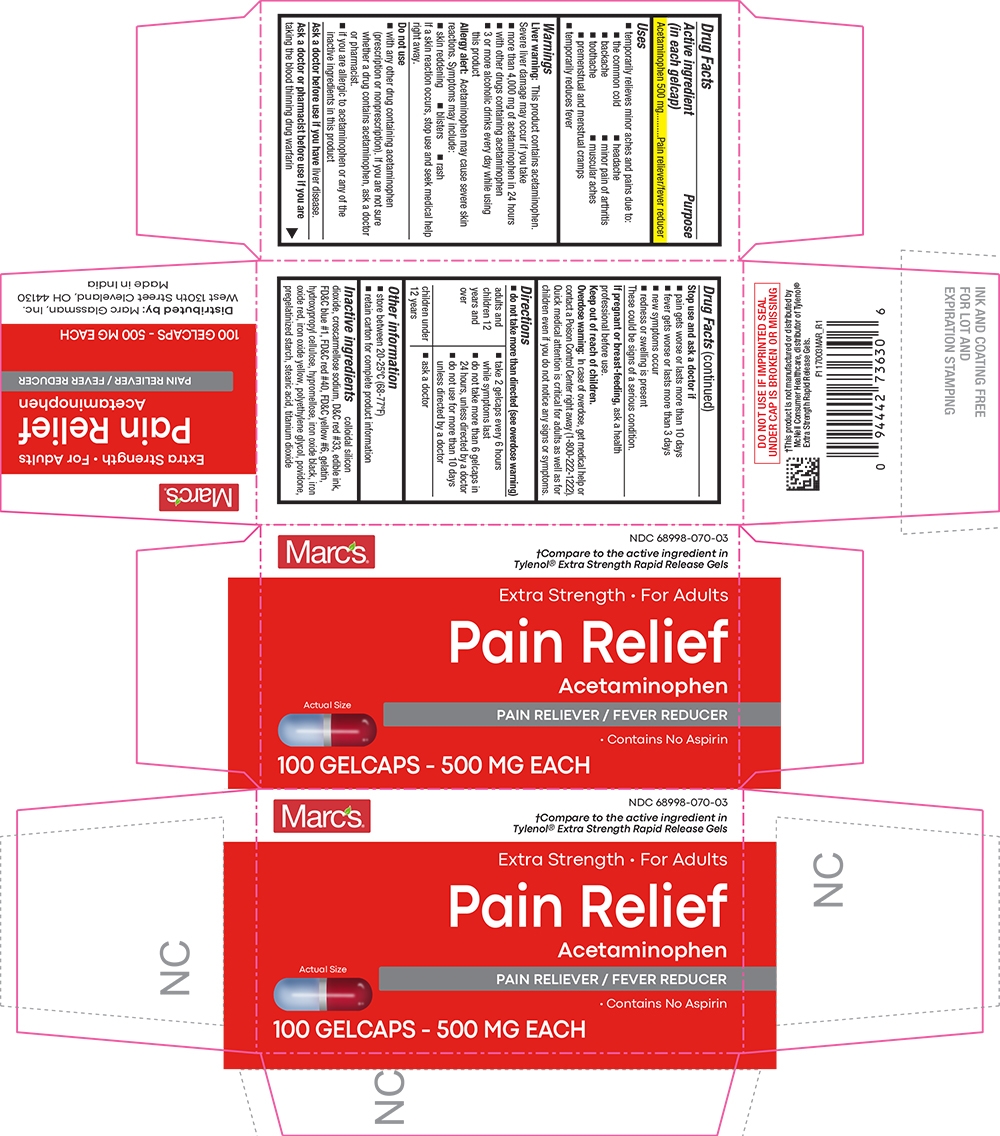
- Active ingredient (in each gelcap)
- Purpose
- Uses
-
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert
acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
-
Directions
- do not take more than directed (see overdose warning)
adults and children 12 years and over - take 2 gelcaps every 6 hours while symptoms last
- do not take more than 6 gelcaps in 24 hours, unless directed by a doctor
- do not use for more than 10 days unless directed by a doctor
children under 12 years - ask a doctor
- Other information
-
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, D&C red #33, edible ink, FD&C blue #1, FD&C red #40, FD&C yellow #6, gelatin, hydroxypropyl cellulose, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, polyethylene glycol, povidone, pregelatinized starch, stearic acid, titanium dioxide
-
PRINCIPAL DISPLAY PANEL
Marc's®
NDC: 68998-070-03
†Compare to the active ingredient in Tylenol® Extra Strength Rapid Release Gels
Extra Strength For Adults
Pain Relief
Acetaminophen
PAIN RELIEVER / FEVER REDUCER
Contains No Aspirin
Actual Size
100 GELCAPS - 500 MG EACH
-
INGREDIENTS AND APPEARANCE
PAIN RELIEF EXTRA STRENGTH
acetaminophen tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68998-070 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSES (UNII: 3NXW29V3WO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color gray (red and light blue ends) Score no score Shape OVAL Size 19mm Flavor Imprint Code G1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68998-070-03 1 in 1 CARTON 09/15/2022 1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 09/15/2022 Labeler - MARC GLASSMAN, INC. (094487477)
Trademark Results [Pain Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PAIN RELIEF 97238381 not registered Live/Pending |
Liu, Caiqing 2022-01-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.